Resveratrol

| |
| |
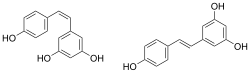 Chemical structures of cis- ((Z)-resveratrol, left) and trans-resveratrol ((E)-resveratrol, right)[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-[(E)-2-(4-Hydroxyphenyl)ethen-1-yl]benzene-1,3-diol | |
| Other names
trans-3,5,4′-Trihydroxystilbene;
3,4′,5-Stilbenetriol; trans-Resveratrol; (E)-5-(p-Hydroxystyryl)resorcinol; (E)-5-(4-hydroxystyryl)benzene-1,3-diol | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
ECHA InfoCard
|
100.121.386 |
| KEGG | |
PubChem CID
|
|
RTECS number
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | white powder with slight yellow cast |
| Melting point | 261 to 263 °C (502 to 505 °F; 534 to 536 K)[2] |
| Solubility in water | 0.03 g/L |
| Solubility in DMSO | 16 g/L |
| Solubility in ethanol | 50 g/L |
| UV-vis (λmax) | 304nm (trans-resveratrol, in water) 286nm (cis-resveratrol, in water)[1] |
| Hazards | |
| GHS labelling:[5] | |

| |
| Warning | |
| H319 | |
| P264, P280, P305+P351+P338, P337+P313 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
23.2 μM (5.29 g)[4] |
| Safety data sheet (SDS) | Fisher Scientific[2] Sigma Aldrich[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a
Although commonly used as a dietary supplement and studied in laboratory models of human diseases,[10] there is no high-quality evidence that resveratrol improves lifespan or has a substantial effect on any human disease.[11][12]
Research
Resveratrol has been studied for its potential therapeutic use,[13] with little evidence of anti-disease effects or health benefits in humans.[6][10][14]
Cardiovascular disease
There is no evidence of benefit from resveratrol in people who already have
Cancer
As of 2020[update], there is no evidence of an effect of resveratrol on cancer in humans.[10][18]
Metabolic syndrome
There is no conclusive evidence for an effect of resveratrol on human metabolic syndrome.[10][19][20] One 2015 review found little evidence for use of resveratrol to treat diabetes.[21] A 2015 meta-analysis found little evidence for an effect of resveratrol on diabetes biomarkers.[22]
One review found limited evidence that resveratrol lowered
Lifespan
As of 2011[update], there is insufficient evidence to indicate that consuming resveratrol has an effect on human lifespan.[11]
Cognition
Resveratrol has been assessed for a possible effect on cognition, but with mixed evidence for an effect. One review concluded that resveratrol had no effect on neurological function, but reported that supplementation improved recognition and mood, although there were inconsistencies in study designs and results.[27]
Diabetes
Although animal experiments have found some evidence that resveratrol may help improve
Other
There is no
Pharmacology
Pharmacodynamics
Resveratrol has been identified as a pan-assay interference compound, which produces positive results in many different laboratory assays.[30] Its ability for varied interactions may be due to direct effects on cell membranes.[31]
As of 2015, many specific biological targets for resveratrol had been identified, including NQO2 (alone and in interaction with AKT1), GSTP1, estrogen receptor beta, CBR1, and integrin αVβ. It was unclear at that time if any or all of these were responsible for the observed effects in cells and model organisms.[32]
Pharmacokinetics
The viability of an oral delivery method is unlikely due to the low aqueous solubility of the molecule. The bioavailability of resveratrol is about 0.5% due to extensive hepatic glucuronidation and sulfation.[33] Glucuronidation occurs in the intestine as well as in the liver, whereas sulfonation not only occurs in the liver but in the intestine and by microbial gut activity.[34] Due to rapid metabolism, the half-life of resveratrol is short (about 8–14 minutes), but the half-life of the sulphate and glucoronide metabolites is above 9 hours.[35]
Metabolism
Resveratrol is extensively metabolized in the body,[6] with the liver and intestines as the major sites of its metabolism.[36][35] Liver metabolites are products of phase II (conjugation) enzymes,[37] which are themselves induced by resveratrol in vitro.[38]
Chemistry
Resveratrol (3,5,4'-trihydroxystilbene) is a stilbenoid, a derivative of
The trans- form can undergo photoisomerization to the cis- form when exposed to ultraviolet irradiation.[40][41]

UV irradiation to cis-resveratrol induces further photochemical reaction, producing a fluorescent molecule named "Resveratrone".[42]
Trans-resveratrol in the powder form was found to be stable under "accelerated stability" conditions of 75% humidity and 40 °C in the presence of air.[43] The trans isomer is also stabilized by the presence of transport proteins.[44] Resveratrol content also was stable in the skins of grapes and pomace taken after fermentation and stored for a long period.[45] lH- and 13C-NMR data for the four most common forms of resveratrols are reported in literature.[39]
Biosynthesis
Resveratrol is produced in plants via the enzyme
Biotransformation
The grapevine fungal
Adverse effects
Only a few human studies have been done to determine the
Occurrences
Plants
Resveratrol is a phytoalexin, a class of compounds produced by many plants when they are infected by pathogens or physically harmed by cutting, crushing, or ultraviolet radiation.[52]
Plants that synthesize resveratrol include
Foods
The levels of resveratrol found in food varies considerably, even in the same food from season to season and batch to batch.[6]
Wine and grape juice
| Beverage | Resveratrol (μg/100 mL)[9] | |
|---|---|---|
| mean | range | |
| Red wine | 270 | 0 — 2780 |
| Rosé wine | 120 | 5 — 290 |
| White wine | 40 | 0 — 170 |
| Sparkling wine | 9 | 8 — 10 |
| Green grape juice | 5.08 | 0 — 10 |
Resveratrol concentrations in red wines average 1.9±1.7 mg trans-resveratrol/L (8.2±7.5 μM), ranging from nondetectable levels to 14.3 mg/L (62.7 μM) trans-resveratrol. Levels of cis-resveratrol follow the same trend as trans-resveratrol.[53]
In general, wines made from grapes of the Pinot noir and St. Laurent varieties showed the highest level of trans-resveratrol, though no wine or region can yet be said to produce wines with significantly higher concentrations than any other wine or region.[53] Champagne and vinegar also contain appreciable levels of resveratrol.[9]
Selected foods
| Food | Serving | Total resveratrol (mg)[6] |
|---|---|---|
| Peanuts (raw) | 1 cup (146 grams) | 0.01 – 0.26 |
| Peanut butter | 1 cup (258 grams) | 0.04 – 0.13 |
| Red grapes | 1 cup (160 grams) | 0.24 – 1.25 |
| Cocoa powder | 1 cup (200 grams) | 0.28 – 0.46 |
Ounce for ounce, peanuts have about 25% as much resveratrol as red wine.[6] Peanuts, especially sprouted peanuts, have a content similar to grapes in a range of 2.3 to 4.5 μg/g before sprouting, and after sprouting, in a range of 11.7 to 25.7 μg/g, depending on peanut cultivar.[9][52]
Most US supplements of resveratrol are derived from the root of Reynoutria japonica (also called Japanese knotweed, Hu Zhang, etc.)[6]
History
The first mention of resveratrol was in a
Related compounds
- Dihydro-resveratrol
- Epsilon-viniferin, Pallidol and Quadrangularin Athree different resveratrol dimers
- Elafibranor, a structurally related compound that acts as a dual PPARα/δ agonist
- He Shou Wuwhich is very similar to resveratrol.
- Trans-diptoindonesin B, a resveratrol trimer
- Hopeaphenol, a resveratrol tetramer
- Oxyresveratrol, the aglycone of mulberroside A, a compound found in Morus alba, the white mulberry[64]
- Piceatannol, an active metabolite of resveratrol found in red wine
- Piceid, a resveratrol glucoside
- Pterostilbene, a doubly methylated resveratrol
- 4'-Methoxy-(E)-resveratrol 3-O-rutinoside, a compound found in the stem bark of Boswellia dalzielii[65]
- Rhaponticin a glucoside of the stilbenoid rhapontigenin, found in rhubarb rhizomes
See also
- Phenolic compounds in wine
- Polyphenol antioxidant
- List of phytochemicals in food
- Phytochemistry
- Secondary metabolites
References
- ^ PMID 19154820.
- ^ a b "Resveratrol MSDS on Fisher Scientific website". Archived from the original on 2012-11-03. Retrieved 2012-03-06.
- ^ Resveratrol MSDS on www.sigmaaldrich.com
- PMID 19207580.
- ^ GHS: Sigma-Aldrich R5010
- ^ a b c d e f g h i j k l m "Resveratrol". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 11 June 2015. Retrieved 26 August 2019.
- PMID 10680575.
- ^ PMID 24579014.
- ^ a b c d "Stilbenes-resveratrol in foods and beverages, version 3.6". Phenol-Explorer. 2016. Retrieved 13 May 2016.
- ^ a b c d e f "Resveratrol". MedlinePlus. 1 April 2019. Retrieved 22 September 2019.
- ^ PMID 21698226.
- PMID 25885871.
- S2CID 73443806.
- PMID 34320173.
- S2CID 206223647.
- ^ S2CID 30351462.
- PMID 24731650.
- PMID 24500760.
- S2CID 206223623.
- PMID 25138371.
- PMID 25446988.
- PMID 25138371.
- PMID 29018489.
- S2CID 54563469.
- S2CID 146806930.
- S2CID 51677307.
- S2CID 4472410.
- PMID 31978258.
- S2CID 201846615.
- PMID 25254460.
- PMID 24901212.
- S2CID 27108183.
- S2CID 10020092.
- S2CID 58651581.
- ^ S2CID 36628503.
- PMID 23474649.
- PMID 30893846.
- PMID 26221416.
- ^ .
- .
- ^ Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon Vol. 84 No. 7 July 2007 Journal of Chemical Education 1159.
- PMID 22436889.
- PMID 16579722.
- PMID 24773207.
- PMID 10051967.
- ^ PMID 33406721.
- ^ S2CID 4015467.
- PMID 28168876.
- PMID 10650073.
- PMID 9784180.
- PMID 23740855.
- ^ S2CID 13183809.
- ^ .
- S2CID 225590316.
- PMID 12840221.
- .
- .
- .
- ^ Rimas A (2006-12-11). "His research targets the aging process". The Boston Globe.
- Fortune magazine.
- ^ Weintraub A (2009-07-29). "Resveratrol: The Hard Sell on Anti-Aging". Bloomberg Businessweek. Archived from the original on July 31, 2009.
- ^ Carroll, John; McBride, Ryan (Mar 12, 2013). "Updated: GSK moves to shutter Sirtris' Cambridge office, integrate R&D". FierceBiotech. Archived from the original on April 28, 2019. Retrieved August 17, 2017.
- ^ "GSK absorbs controversial 'longevity' company: News blog". Nature Blog. Archived from the original on 2013-12-17. Retrieved 2017-08-17..
- S2CID 21236818.
- ^ Alemika Taiwo E, Onawunmi Grace O and Olugbade Tiwalade O, Antibacterial phenolics from Boswellia dalzielii. Nigerian Journal of Natural Products and Medicines, 2006
External links
 Media related to Resveratrol at Wikimedia Commons
Media related to Resveratrol at Wikimedia Commons
