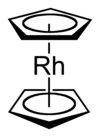
Rhodocene
| |

| |
| Names | |
|---|---|
| IUPAC name
Rhodocene
| |
Other names
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H10Rh | |
| Molar mass | 233.095 g·mol−1 |
| Appearance | yellow solid (dimer)[1] |
| Melting point | 174 °C (345 °F; 447 K) with decomposition (dimer)[1] |
| |
| Related compounds | |
Related compounds
|
iridocene, bis(benzene)chromium
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rhodocene is a
The history of
Owing to their stability and relative ease of preparation, rhodocenium salts are the usual starting material for preparing rhodocene and substituted rhodocenes, all of which are unstable. The original synthesis used a cyclopentadienyl anion and tris(acetylacetonato)rhodium(III);[11] numerous other approaches have since been reported, including gas-phase redox transmetalation[16] and using half-sandwich precursors.[17] Octaphenylrhodocene (a derivative with eight phenyl groups attached) was the first substituted rhodocene to be isolated at room temperature, though it decomposes rapidly in air. X-ray crystallography confirmed that octaphenylrhodocene has a sandwich structure with a staggered conformation.[18] Unlike cobaltocene, which has become a useful one-electron reducing agent in research,[19] no rhodocene derivative yet discovered is stable enough for such applications.
History
Discoveries in
10H
10Fe and reported to exhibit "remarkable stability".[10] The discovery sparked substantial interest in the field of organometallic chemistry,[8][9] in part because the structure proposed by Pauson and Kealy was inconsistent with then-existing bonding models and did not explain its unexpected stability. Consequently, the initial challenge was to definitively determine the structure of ferrocene in the hope that its bonding and properties would then be understood. The sandwich structure was deduced and reported independently by three groups in 1952: Robert Burns Woodward and Geoffrey Wilkinson investigated the reactivity in order to determine the structure[30] and demonstrated that ferrocene undergoes similar reactions to a typical aromatic molecule (such as benzene),[31] Ernst Otto Fischer deduced the sandwich structure and also began synthesising other metallocenes including cobaltocene;[32] Eiland and Pepinsky provided X-ray crystallographic confirmation of the sandwich structure.[33] Applying valence bond theory to ferrocene by considering an Fe2+
centre and two cyclopentadienide anions (C5H5−), which are known to be aromatic according to Hückel's rule and hence highly stable, allowed correct prediction of the geometry of the molecule. Once molecular orbital theory was successfully applied, the reasons for ferrocene's remarkable stability became clear.[13]
The properties of cobaltocene reported by Wilkinson and Fischer demonstrated that the unipositive cobalticinium cation [Co(C5H5)2]+ exhibited stability similar to that of ferrocene itself. This observation is not unexpected given that the cobalticinium cation and ferrocene are
The stability of metallocenes can be directly compared by looking at the
- [Fe(C5H5)2]+ / [Fe(C5H5)2] +0.38 V[34]
- [Co(C5H5)2]+ / [Co(C5H5)2] −0.94 V[1]
- [Rh(C5H5)2]+ / [Rh(C5H5)2] −1.41 V[1]
These data clearly indicate the stability of neutral ferrocene and the cobaltocenium and rhodocenium cations. Rhodocene is ca. 500 mV more reducing than cobaltocene, indicating that it is more readily oxidised and hence less stable.
Speciation
The
Rhodocene exists as [Rh(C5H5)2], a
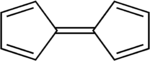
This dimerisation process has the overall effect of decreasing the electron count around the rhodium centre from 19 to 18. This occurs because the oxidative coupling of the two cyclopentadienyl ligands produces a new ligand with lower hapticity and which donates fewer electrons to the metal centre. The term hapticity is used to indicate the "number of carbon (or other) atoms through which [a ligand] binds (n)"[39] to a metal centre and is symbolised as ηn. For example, the ethylene ligand in Zeise's salt is bound to the platinum centre through both carbon atoms, and it hence formally has the formula K[PtCl3(η2-C2H4)]·H2O.[6] The carbonyl ligands in nickel tetracarbonyl are each bound through only a carbon atom and are hence described as monohapto ligands, but η1-notations are typically omitted in formulae. The cyclopentadienyl ligands in many metallocene and half-sandwich compounds are pentahapto ligands, hence the formula [Rh(η5-C5H5)2] for the rhodocene monomer. In the rhodocene dimer, the coupled cyclopentadienyl ligands are 4-electron tetrahapto donors to each rhodium(I) metal centre, in contrast to the 6-electron[Note 4] pentahapto cyclopentadienyl donors. The increased stability of the 18-valence electron rhodium(I) dimer species as compared to the 19-valence electron rhodium(II) monomer likely explains why the monomer is only detected under extreme conditions.[1][4]

Cotton and Wilkinson demonstrated[11] that the 18-valence electron rhodium(III) rhodocenium cation [Rh(η5-C5H5)2]+ can be reduced in aqueous solution to the monomeric form; they were unable to isolate the neutral product as not only can it dimerise, the rhodium(II) radical monomer can also spontaneously form the mixed-hapticity stable rhodium(I) species [(η5-C5H5)Rh(η4-C5H6)].[3] The differences between rhodocene and this derivative are found in two areas:
- One of the bound cyclopentadienyl ligands has formally gained a hydrogen atom to become cyclopentadiene, which remains bound to the metal centre but now as a 4-electron η4- donor.
- The rhodium(II) metal centre has been reduced to rhodium(I).
These two changes make the derivative an 18-valence electron species. Fischer and colleagues hypothesised that the formation of this rhodocene derivative might occur in separate protonation and reduction steps, but published no evidence to support this suggestion.[3] (η4-Cyclopentadiene)(η5-cyclopentadienyl)rhodium(I), the resulting compound, is an unusual organometallic complex in that it has both a cyclopentadienyl anion and cyclopentadiene itself as ligands. It has been shown that this compound can also be prepared by sodium borohydride reduction of a rhodocenium solution in aqueous ethanol; the researchers who made this discovery characterised the product as biscyclopentadienylrhodium hydride.[40]
Fischer and co-workers also studied the chemistry of iridocene, the third transition series analogue of rhodocene and cobaltocene, finding the chemistry of rhodocene and iridocene are generally similar. The synthesis of numerous iridocenium salts including the tribromide and hexafluorophosphate have been described.[4] Just as with rhodocene, iridocene dimerises at room temperature but a monomer form can be detected at low temperatures and in gas phase and IR, NMR, and ESR measurements indicate a chemical equilibrium is present and confirm the sandwich structure of the iridocene monomer.[3][4] The complex [(η5-C5H5)Ir(η4-C5H6)], the analogue of rhodocene derivative reported by Fischer,[3] has also been studied and demonstrates properties consistent with a greater degree of π-backbonding in iridium(I) systems than is found in the analogous cobalt(I) or rhodium(I) cases.[41]
Synthesis
Rhodocenium salts were first reported[11] within two years of the discovery of ferrocene.[10] These salts were prepared by reacting the carbanion Grignard reagent cyclopentadienylmagnesium bromide (C
5H
5MgBr) with tris(acetylacetonato)rhodium(III) (Rh(acac)3). More recently, gas-phase rhodocenium cations have been generated by a redox transmetalation reaction of rhodium(I) ions with ferrocene or nickelocene.[16]
- Rh+ + [(η5-C5H5)2M] → M + [(η5-C5H5)2Rh]+ M = Ni or Fe
Modern
Rhodocene itself is then formed by reduction of rhodocenium salts with molten sodium.[3] If a rhodocenium containing melt is treated with sodium or potassium metals and then sublimed onto a liquid nitrogen-cooled cold finger, a black polycrystalline material results.[36] Warming this material to room temperature produces a yellow solid which has been confirmed as the rhodocene dimer. A similar method can be used to prepare the iridocene dimer.[36]
Substituted rhodocenes and rhodocenium salts
The [(η5-C5tBu3H2)Rh(η5-C5H5)]+ cation
Novel approaches to synthesising substituted cyclopentadienyl complexes have been developed using substituted vinylcyclopropene starting materials.

The 18-valence electron rhodium(III) pentadienediyl species generated by this reaction demonstrates again the instability of the rhodocene moiety, in that it can be refluxed in toluene for months without 1,2,3-tri-tert-butylrhodocene forming but in oxidising conditions the 1,2,3-tri-tert-butylrhodocenium cation forms rapidly.[44] Cyclic voltammetry has been used to investigate this and similar processes in detail.[44][45] The mechanism of the reaction has been shown to involve a loss of one electron from the pentadienediyl ligand followed by a fast rearrangement (with loss of a hydrogen atom) to form the 1,2,3-tri-tert-butylrhodocenium cation.[45] Both the tetrafluoroborate and hexafluorophosphate salts of this cation have been structurally characterised by X-ray crystallography.[45]
[(η5-C5tBu3H2)Rh(η5-C5H5)]BF4 forms a colourless

The diagram above shows the rhodium–carbon (in red, inside pentagons on the left) and carbon–carbon (in blue, outside pentagons on the left) bond distances for both ligands, along with the bond angles (in green, inside pentagons on the right) within each cyclopentadienyl ring. The atom labels used are the same as those shown in the crystal structure above. Within the unsubstituted cyclopentadienyl ligand, the carbon–carbon bond lengths vary between 1.35 Å and 1.40 Å and the internal bond angles vary between 107° and 109°. For comparison, the internal angle at each vertex of a
The stability of metallocenes changes with ring substitution. Comparing the reduction potentials of the cobaltocenium and decamethylcobaltocenium cations shows that the decamethyl species is ca. 600 mV more reducing than its parent metallocene,
| Half-reaction | E° (V) |
|---|---|
| [Fe(C5H5)2]+ + e− ⇌ [Fe(C5H5)2] | 0 (by definition) |
| [Fe(C5Me5)2]+ + e− ⇌ [Fe(C5Me5)2] | −0.59[48] |
| [Co(C5H5)2]+ + e− ⇌ [Co(C5H5)2] | −1.33[19] |
| [Co(C5Me5)2]+ + e− ⇌ [Co(C5Me5)2] | −1.94[19] |
| [Rh(C5H5)2]+ + e− ⇌ [Rh(C5H5)2] | −1.79[1] † |
| [Rh(C5Me5)2]+ + e− ⇌ [Rh(C5Me5)2] | −2.38[49] |
| [(C5 tBu 3H2)Rh(C5H5)] |
−1.83[45] |
| [(C5tBu3H2)Rh(C5Me5)]+ + e− ⇌ [(C5tBu3H2)Rh(C5Me5)] | −2.03 [45] |
| [(C5H5Ir(C5Me5)]+ + e− ⇌ [(C5H5Ir(C5Me5)] | −2.41[51] † |
| [Ir(C5Me5)2]+ + e− ⇌ [Ir(C5Me5)2] | −2.65[51] † |
| † after correcting by 0.38 V[34] for the different standard |
The differences in reduction potentials are attributed in the cobaltocenium system to the inductive effect of the alkyl groups,[19] further stabilising the 18-valence electron species. A similar effect is seen in the rhodocenium data shown above, again consistent with inductive effects.[45] In the substituted iridocenium system, cyclic voltammetry investigations shows irreversible reductions at temperatures as low as −60 °C;[51] by comparison, the reduction of the corresponding rhodocenes is quasi-reversible at room temperature and fully reversible at −35 °C.[49] The irreversibility of the substituted iridocenium reductions is attributed to the extremely rapid dimerisation of the resulting 19-valence electron species, which further illustrates that iridocenes are less stable than their corresponding rhodocenes.[51]
Pentasubstituted cyclopentadienyl ligands
The body of knowledge concerning compounds with penta-substituted cyclopentadienyl ligands is extensive, with organometallic
In a similar reaction, pentaisopropylrhodocenium hexafluorophosphate [(η5-C5iPr5)Rh(η5-C5H5)]PF6 can be synthesised from pentamethylrhodocenium hexafluorophosphate [(η5-C5Me5)Rh(η5-C5H5)]PF6 in 80% yield.
The compounds pentaphenylrhodocenium tetrafluoroborate [(η5-C5Ph5)Rh(η5-C5H5)]BF4, and pentamethylpentaphenylrhodocenium tetrafluoroborate [(η5-C5Ph5)Rh(η5-C5Me5)]BF4 have also been reported. They demonstrate that rhodium sandwich compounds can be prepared from half-sandwich precursors. For example, in an approach broadly similar to the tris(acetone) synthesis of decamethylrhodocenium tetrafluoroborate,[55] pentaphenylrhodocenium tetrafluoroborate has been synthesised from the tris(acetonitrile) salt [(η5-C5Ph5)Rh(CH3CN)3](BF4)2 by reaction with sodium cyclopentadienide:[17]
- [(η5-C5Ph5)Rh(MeCN)3](BF4)2 + NaC5H5 → [(η5-C5Ph5)Rh(η5-C5H5)]BF4 + NaBF4 + 3 MeCN

Octaphenylrhodocene, [(η5-C5Ph4H)2Rh], is the first rhodocene derivative to be isolated at room temperature. Its olive-green crystals decompose rapidly in solution, and within minutes in air, demonstrating a dramatically greater air sensitivity than the analogous
The synthesis of octaphenylrhodocene proceeds in three steps, with a diglyme reflux followed by workup with hexafluorophosphoric acid, then a sodium amalgam reduction in tetrahydrofuran:[18]
- Rh(acac)3 + 2 KC5Ph4H → [(η5-C5Ph4H)2Rh]+ + 2 K+ + 3 acac−
- [(η5-C5Ph4H)2Rh]+ + 3 acac− + 3 HPF6 → [(η5-C5Ph4H)2Rh]PF6 + 3 Hacac + 2 PF6−
- [(η5-C5Ph4H)2Rh]PF6 + Na/Hg → [(η5-C5Ph4H)2Rh] + NaPF6
The crystal structure of octaphenylrhodocene shows a staggered conformation[18] (similar to that of ferrocene, and in contrast to the eclipsed conformation of ruthenocene).[13] The rhodium–centroid distance is 1.904 Å and the rhodium–carbon bond lengths average 2.26 Å; the carbon–carbon bond lengths average 1.44 Å.[18] These distances are all similar to those found in the 1,2,3-tri-tert-butylrhodocenium cation described above, with the one difference that the effective size of the rhodium centre appears larger, an observation consistent with the expanded ionic radius of rhodium(II) compared with rhodium(III).[45]
Applications
Biomedical use of a derivative
There has been extensive research into
Metal–metal interactions in linked metallocenes

The original motivation for research investigations of the rhodocene system was to understand the nature of and bonding within the metallocene class of compounds. In more recent times, interest has been rekindled by the desire to explore and apply the metal–metal interactions that occur when metallocene systems are linked.
Structural studies of termetallocene systems have shown they typically adopt an "eclipsed double transoid" "crankshaft" geometry.[62] Taking as an example the 1-cobaltocenyl-1'-rhodocenylferrocene cation shown above, this means that the cobaltocenyl and rhodocenyl moieties are eclipsed, and thus carbon atoms 1 and 1' on the central ferrocene core are as close to vertically aligned as is possible given the staggered conformation of the cyclopentadienyl rings within each metallocene unit. Viewed from side-on, this means termetallocenes resemble the down–up–down pattern of a crankshaft.[62] The synthesis of this termetallocene involves the combining of rhodocenium and cobaltocenium solutions with 1,1'-dilithioferrocene. This produces an uncharged intermediate with linked cyclopentadienyl–cyclopentadiene ligands whose bonding resembles that found in the rhodocene dimer. These ligands then react with the triphenylmethyl carbocation to generate the termetallocene salt, [(η5-C5H5)Rh(μ-η5:η5-C5H4–C5H4)Fe(μ-η5:η5-C5H4–C5H4)Co(η5-C5H5)](PF6)2. This synthetic pathway is illustrated below:[61][62]
Rhodocenium-containing polymers
The first rhodocenium-containing side-chain polymers were prepared through controlled polymerization techniques such as reversible addition−fragmentation chain-transfer polymerization (RAFT) and ring-opening metathesis polymerisation (ROMP).[63]
Notes
- ^ a b The 18-valence electron cation [Rh(C5H5)2]+ is called the rhodocenium cation in some journal articles[1] and the rhodicinium cation in others.[11] The former spelling appears more common in more recent literature and so is adopted in this article, but both formulations refer to the same chemical species.
- ^ The presence of a mirror plane perpendicular to the C
5 ring centroid–metal–ring centroid axis of symmetry suggests an eclipsed rather than a staggered conformation. Free rotation of cyclopentadienyl ligands about this axis is common in metallocenes – in ferrocene, the energy barrier to rotation is ~5 kJ mol−1.[13] Consequently, there would be both staggered and eclipsed rhodocene monomer molecules co-existing, and rapidly interconverting, in the solution. It is only in the solid state that a definitive assignment of staggered or eclipsed conformation is meaningful. - ^ In the rhodocene dimer, the joined cyclopentadiene rings are shown with the H atoms in the "endo" position (i.e. the H's are inside, the other half of the ligands are on the outside). Although this is not based on crystal structure data, it does follow the illustrations provided by El Murr et al.[1] and by Fischer and Wawersik[3] in their discussion of the 1H NMR data they collected. The paper by Collins et al.,[18] shows the H atoms in the "exo" position.
- ^ There are two distinct approaches to electron counting, based on either radical species or ionic species. Using the radical approach, a rhodium centre has 9 electrons irrespective of its oxidation states and a cyclopentadienyl ligand is a 5 electron donor. Using the ionic approach, the cyclopentadienyl ligand is a 6 electron donor and the electron count of the rhodium centre depends on its oxidation state – rhodium(I) is an 8 electron centre, rhodium(II) is a 7 electron centre, and rhodium(III) is a 6 electron centre. The two approaches generally reach the same conclusions but it is important to be consistent in using only one or the other.
- tert-butylgroup, —C(CH3)3.
References
- ^ .
- ^ ISBN 978-0-470-25762-3.
An industrial application of transition metal organometallic chemistry appeared as early as the 1880s, when Ludwig Mond showed that nickel can be purified by using CO to pick up nickel in the form of gaseous Ni(CO)4 that can easily be separated from solid impurities and later be thermally decomposed to give pure nickel.
... Recent work has shown the existence of a growing class of metalloenzymes having organometallic ligand environments – considered as the chemistry of metal ions having C-donor ligands such as CO or the methyl group
- ^ .
- ^ .
- ^ from the original on 6 August 2020. Retrieved 12 September 2020.
- ^ Platinum Metals Review. 28 (2): 76–83. Archived(PDF) from the original on 24 September 2015. Retrieved 8 January 2011.
- ^ ISBN 9780854044696. Archivedfrom the original on 26 January 2020. Retrieved 17 June 2017.
- ^ PMID 10649350.
- ^ doi:10.1002/chin.200443242. (Abstract; original published in Trends in Organometallic Chemistry, 4:147–169, 2002)
- ^ S2CID 4181383.
- ^ .
- ^ .
- ^ ISBN 978-81-224-1258-1. Archivedfrom the original on 7 December 2016. Retrieved 15 July 2016.
- ^ a b "The Nobel Prize in Chemistry 1973". Nobel Foundation. Archived from the original on 25 October 2012. Retrieved 12 September 2010.
- ^ a b Sherwood, Martin (1 November 1973). "Metal Sandwiches". New Scientist. 60 (870): 335. Archived from the original on 3 November 2021. Retrieved 17 June 2017.
- ^ .
- ^ )
- ^ .
- ^ PMID 11848774.
- ^ ISBN 0-470-86403-6.
- ^ PMID 2851003.
- ^ PMID 3030970.
- ^ PMID 11848884.
- ^ PMID 16906602.
- ^ .
- ^ .
- ^ ISBN 0-85404-469-8. Archivedfrom the original on 7 December 2016. Retrieved 15 July 2016.
- .
- ^ ISBN 978-3-540-46128-9. Archivedfrom the original on 15 February 2021. Retrieved 15 July 2016.
- .
- ISBN 978-0-387-09847-0. Archivedfrom the original on 7 December 2016. Retrieved 15 July 2016.
- ^ .
- .
- ^ .
- ^ ISBN 978-0-495-38703-9.
- ^ ISBN 978-0-471-68242-4.
- ^ .
- ^ ISBN 0-7514-0413-6. Archivedfrom the original on 29 July 2014. Retrieved 15 July 2016.
Both metals exhibit an extensive chemistry, principally in the +3 oxidation state, with +1 also being important, and a significant chemistry of +4 iridium existing. Few compounds are known in the +2 state, in contrast to the situation for cobalt, their lighter homologue (factors responsible include the increased stability of the +3 state consequent upon the greater stabilization of the low spin d6 as 10 Dq increases)." (p. 78)
- ISBN 0-85404-622-4.
- .
- .
- .
- .
- ^ .
- ^ .
- .
- .
- ^ .
- ^ .
- .
- ^ .
- ISBN 3-540-54324-4.
- S2CID 95222717.
- ^ .
- ^ .
- .
- ISBN 3-540-65308-2.
- ISBN 978-0-85404-596-9.
- .
- doi:10.1002/aoc.1202.
- ^ S2CID 93898229.
- ^ .
- from the original on 3 November 2021. Retrieved 4 May 2021.



![{\displaystyle {\ce {{RhCl3.{\mathit {x}}H2O}+{2C5H6}+NH4PF6->{[(\eta ^{5}-C5H5)2Rh]PF6}(v)+{2HCl}+{NH4Cl}+{\mathit {x}}H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/afc634acfe3aa0ac2cc66328dffe2923c224cf6a)


