Sharpless epoxidation
| Sharpless epoxidation | |
|---|---|
| Named after | Karl Barry Sharpless |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | sharpless-epoxidation |
| RSC ontology ID | RXNO:0000141 |
The Sharpless epoxidation reaction is an
2,3-Epoxyalcohols can be converted into diols, aminoalcohols, and ethers. The reactants for the Sharpless epoxidation are commercially available and relatively inexpensive.[6]
Catalyst
5–10 mol% of the catalyst is typical. The presence of 3Å molecular sieves (3Å MS) is necessary.[7] The structure of the catalyst is uncertain although it is thought to be a dimer of [Ti(tartrate)(OR)2].[8]
Selectivity
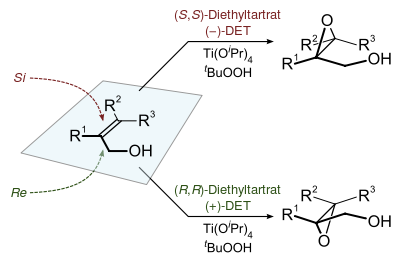
The epoxidation of allylic alcohols is a well-utilized conversion in fine chemical synthesis. The chirality of the product of a Sharpless epoxidation is sometimes predicted with the following mnemonic. A rectangle is drawn around the double bond in the same plane as the carbons of the double bond (the xy-plane), with the allylic alcohol in the bottom right corner and the other substituents in their appropriate corners. In this orientation, the (−) diester tartrate preferentially interacts with the top half of the molecule, and the (+) diester tartrate preferentially interacts with the bottom half of the molecule. This model seems to be valid despite substitution on the olefin. Selectivity decreases with larger R1, but increases with larger R2 and R3 (see introduction).[1]

However, this method incorrectly predicts the product of allylic 1,2-diols.[9]

Kinetic resolution
The Sharpless epoxidation can also give kinetic resolution of a racemic mixture of secondary 2,3-epoxyalcohols. While the yield of a kinetic resolution process cannot be higher than 50%, the enantiomeric excess approaches 100% in some reactions.[10][11]

Synthetic utility
The Sharpless epoxidation is viable with a large range of primary and secondary alkenic alcohols. Furthermore, with the exception noted above, a given dialkyl tartrate will preferentially add to the same face independent of the substitution on the alkene.To demonstrate the synthetic utility of the Sharpless epoxidation, the Sharpless group created synthetic intermediates of various natural products: methymycin, erythromycin, leukotriene C-1, and (+)-disparlure.[12]

As one of the few highly enantioselective reactions during its time, many manipulations of the 2,3-epoxyalcohols have been developed.[13]
The Sharpless epoxidation has been used for the total synthesis of various
The main drawback of this protocol is the necessity of the presence of an
References of historic interest
- Katsuki, T.; .
- Gao, Y.; Hanson, R. M.; Klunder, J. M.; Ko, S. Y.; Masamune, H.; .
See also
- Asymmetric catalytic oxidation
- enones
- Jacobsen epoxidation — for unfunctionalized alkenes
References
- ^ PMID 16771446.
- ISBN 978-0-08-052349-1.
- ISBN 978-3-540-56252-8.
- ISBN 0471264180.
- .
- ^ .
- .
- .
- .
- .
- .
- .
- .
