Snake venom
Snake venom is a highly toxic
The glands that secrete zootoxins are a modification of the
Venom contains more than 20 different compounds, which are mostly proteins and
Chemistry
| Type | Name | Origin |
|---|---|---|
| Oxidoreductases | lactate dehydrogenase | Elapidae |
| L-amino-acid oxidase | All species | |
| Catalase | All species | |
| Transferases | Alanine amino transferase |
|
| Hydrolases | Phospholipase A2 | All species |
| Lysophospholipase | Elapidae, Viperidae | |
| Acetylcholinesterase | Elapidae | |
| Alkaline phosphatase | Bothrops atrox | |
| Acid phosphatase | Deinagkistrodon acutus
| |
| 5'-nucleotidase | All species | |
| Phosphodiesterase | All species | |
| Deoxyribonuclease | All species | |
| Ribonuclease 1 | All species | |
| Adenosine triphosphatase | All species | |
| Amylase | All species | |
| Hyaluronidase | All species | |
| NAD-Nucleotidase | All species | |
| Kininogenase | Viperidae | |
| Factor X activator | Viperidae, Crotalinae | |
| Heparinase | Crotalinae | |
| α-Fibrinogenase | Viperidae, Crotalinae | |
| β-Fibrinogenase | Viperidae, Crotalinae | |
| α-β-Fibrinogenase | Bitis gabonica
| |
| Fibrinolytic enzyme | Crotalinae | |
| Prothrombin activator | Crotalinae | |
| Collagenase | Viperidae | |
| Elastase | Viperidae | |
| Lyases | Glucosaminate ammonia-lyase |
Snake toxins vary greatly in their functions. The two broad classes of toxins found in snake venoms are
| α-neurotoxins | α-Bungarotoxin, α-toxin, erabutoxin, cobratoxin
|
|---|---|
| β-neurotoxins (PLA2) | |
| κ-neurotoxins | Kappa-bungarotoxin
|
| Dendrotoxins (Kunitz) | β-Bungarotoxin chain B
|
| Cardiotoxins | Naja nigricollis y-toxin, cardiotoxin III (aka cytotoxins) |
| Myotoxins | Myotoxin-a, crotamine |
| Sarafotoxins | Sarafotoxins a, b, and c |
| Hemorrhagins (metalloprotease) | Mucrolysin, Atrolysins, Acutolysins, etc.[11] |
| Hemotoxins (serine protease) | Venombin A |
Toxins
This section is missing information about how evolutionary/structural classifications correspond to functional classifications. (August 2021) |
Neurotoxins
The beginning of a new neural impulse goes as follows:
- An exchange of ions (charged atoms) across the nerve cell membrane sends a depolarizing current towards the end of the nerve cell (cell terminus).
- When the depolarizing current arrives at the nerve cell terminus, the neurotransmitter acetylcholine (ACh), which is held in vesicles, is released into the space between the two nerves (synapse). It moves across the synapse to the postsynaptic receptors.
- ACh binds to the receptors and transfers the signal to the target cell, and after a short time, it's destroyed by acetylcholinesterase.
Fasciculins
- These toxins attack mice, they cause severe, generalized and long-lasting (5-7 h) fasciculations(rapid muscle contractions).
- Snake example: found mostly in the venom of mambas (Dendroaspis spp.) and some rattlesnakes (Crotalus spp.)
Dendrotoxins
- Dendrotoxins inhibit neurotransmissions by blocking the exchange of positive and negative ions across the neuronal membrane lead to no nerve impulse, thereby paralyzing the nerves.
- Snake example: mambas
α-neurotoxins
- Alpha-neurotoxins are a large group; over 100 postsynaptic neurotoxins having been identified and sequenced.[12] α-neurotoxins attack the Nicotinic acetylcholine receptorsof cholinergic neurons. They mimic the shape of the acetylcholine molecule, and so fit into the receptors, where they block the ACh flow, leading to a feeling of numbness and paralysis.
- Snake examples: cobras (Naja spp.) (known as cobratoxin)
Cytotoxins

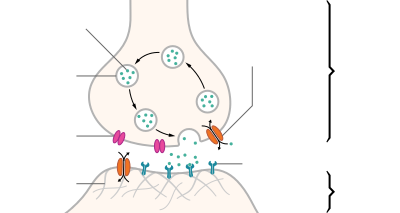
Phospholipases
- Phospholipase is an enzyme that transforms the phospholipid molecule into a lysophospholipid (soap) → the new molecule attracts and binds fat and ruptures cell membranes. Phospholipase A2 is one specific type of phospholipases found in snake venom.
- Snake example: Okinawan habu(Trimeresurus flavoviridis)
Cardiotoxins / Cytotoxins
- Cardiotoxins are components that are specifically toxic to the heart. They bind to particular sites on the surface of muscle cells and cause depolarisation → the toxin prevents muscle contraction. These toxins may cause the heart to beat irregularly or stop beating, causing death. An example is the three-fingered cardiotoxin III from Chinese cobra, an example of the short three-fingered family (InterPro: IPR003572).
- Snake example: mambas, and some Naja species
Hemotoxins
- Hemotoxins cause hemolysis, the destruction of red blood cells (erythrocytes), or induce blood coagulation (clotting, e.g. mucrocetin). A common family of hemotoxins includes snake venom metalloproteinases such as mucrolysin.[11][14]
- Snake examples: most vipers and many cobra species: The tropical rattlesnake Crotalus durissus produces convulxin, a coagulant.[15]
Myotoxins
The first myotoxin to be identified and isolated was
Determining venom toxicity (LD50)
This section is missing information about why albumin dilution works better. (August 2021) |
Snake venom toxicity is assessed by a toxicological test called the
Several techniques have been designed to this end. One approach is to use 0.1% bovine serum albumin (also known as "fraction V" in
When the ultimate goal of plasma processing is a purified plasma component for
However, chromatographic methods began to be adopted in the 1980s.[
- smooth automation and a relatively inexpensive plant was needed,
- easier to sterilize equipment and maintain a good manufacturing environment
- chromatographic processes are less damaging to the albumin protein
- a more successful albumin result can be achieved.[citation needed]
Compared with the Cohn process, albumin purity increased from about 95% to 98% using chromatography, and the yield increased from about 65% to 85%. Small percentage increases make a difference in regard to sensitive measurements such as purity. The big drawback has to do with the economics. Although the method offered efficient, acquiring the necessary equipment was difficult. Large machinery is necessary, and for a long time, the lack of equipment availability limited its widespread use.[citation needed]
Evolution
Venom evolved just once among all
The mechanism of evolution in most cases has been gene duplication in tissues unrelated to the venom.[24] Pre-existing salivary proteins are the likely ancestors of most venom toxin genes.[26] Expression of the new protein in the venom gland followed duplication.[24] Then proceeded natural selection for adaptive traits following the birth-and-death model, where duplication is followed by functional diversification, resulting in the creation of structurally related proteins that have slightly different functions.[23][24][27] The study of venom evolution has been a high priority for scientists in terms of scientific research, due to the medical relevance of snake venom, in terms of making antivenom and cancer research. Knowing more about the composition of venom and the ways it can potentially evolve is very beneficial. Three main factors that affect venom evolution have been closely studied: predators of the snake that are resistant to snake venom, prey that are in an evolutionary arms race with snakes, and the specific diets that affect the intraspecific evolution of venom.[23][28] Venoms continue to evolve as specific toxins and are modified to target a specific prey, and toxins are found to vary according to diet in some species.[29][30]
Rapid venom evolution can also be explained by the arms race between venom-targeted molecules in resistant predators, such as the opossum, and the snake venom that targets the molecules. Scientists performed experiments on the opossums and found that multiple trials showed replacement to silent substitutions in the von Willebrand factor (vWf) gene that encodes for a venom-targeted hemostatic blood protein. These substitutions are thought to weaken the connection between vWf and a toxic snake venom ligand (botrocetin), which changes the net charge and hydrophobicity. These results are significant to the venom evolution because it's the first citation of rapid evolution in a venom-targeted molecule. This shows that an evolutionary arms race may be occurring in terms of defensive purposes. Alternative hypotheses suggest that venom evolution is due to trophic adaption, whereas these scientists believe, in this case, that selection would occur on traits that help with prey survival in terms of venom evolution instead of predation success. Several other predators of the pit viper (mongooses and hedgehogs) show the same type of relationship between snakes, which helps to support the hypothesis that venom has a very strong defensive role along with a trophic role. Which in turn supports the idea that predation on the snakes can be the arms race that produces snake venom evolution.[31]
Some of the various adaptations produced by this process include venom more toxic to specific prey in several lineages,
Injection
Vipers
In
Elapids
In the
Colubrids
Mechanics of biting
Several genera, including Asian coral snakes (Calliophis), burrowing asps (Atractaspis), and night adders (Causus), are remarkable for having exceptionally long venom glands, extending along each side of the body, in some cases extending posterially as far as the heart. Instead of the muscles of the temporal region serving to press out the venom into the duct, this action is performed by those of the side of the body.
Considerable variability in biting behavior is seen among snakes. When biting, viperid snakes often strike quickly, discharging venom as the fangs penetrate the skin, and then immediately release. Alternatively, as in the case of a feeding response, some viperids (e.g. Lachesis) bite and hold. A
Differences in fang length between the various venomous snakes are likely due to the evolution of different striking strategies.[39] Additionally, it has been shown that the fangs of different species of venomous snakes have different sizes and shapes depending on the biomechanical properties of the snake's prey.[40]
Mechanics of spitting
Spitting is a defensive reaction only. The snakes tend to aim for the eyes of a perceived threat. A direct hit can cause temporary shock and blindness through severe inflammation of the cornea and conjunctiva. Although usually no serious symptoms result if the venom is washed away immediately with plenty of water, blindness can become permanent if left untreated. Brief contact with the skin is not immediately dangerous, but open wounds may be vectors for envenomation.
Physiological effects
This article needs additional citations for verification. (August 2021) |
The four distinct types of venom act on the body differently:
- Proteolyticvenom dismantles the molecular surroundings, including at the site of the bite.
- Hemotoxicvenom acts on the cardiovascular system, including the heart and blood.
- Neurotoxicvenom acts on the nervous system, including the brain.
- Cytotoxicvenom has a localized action at the site of the bite.
Proteroglyphous snakes
The effect of the venom of
Vipers
Viper venom (
The bite is immediately followed by the local pain of a burning character; the limb soon swells and becomes discolored, and within one to three hours great prostration, accompanied by
suppuration. That cases of death, in adults as well as in children, are not infrequent in some parts of the Continent is mentioned in the last chapter of this Introduction.
The Viperidae differ much among themselves in the toxicity of their venoms. Some, such as the Indian Russell's viper (Daboia russelli) and saw-scaled viper (E. carinatus); the American rattlesnakes (Crotalus spp.), bushmasters (Lachesis spp.), and lanceheads (Bothrops spp.); and the African adders (Bitis spp.), night adders (Causus spp.), and horned vipers (Cerastes spp.), cause fatal results unless a remedy is speedily applied. The bite of the larger European vipers may be very dangerous, and followed by fatal results, especially in children, at least in the hotter parts of the Continent; whilst the small meadow viper (Vipera ursinii), which hardly ever bites unless roughly handled, does not seem to be possessed of a very virulent venom, and although very common in some parts of Austria and Hungary, is not known to have ever caused a serious accident.
Opisthoglyphous colubrids
Biologists had long known that some snakes had rear fangs, 'inferior' venom injection mechanisms that might immobilize prey; although a few fatalities were on record, until 1957, the possibility that such snakes were deadly to humans seemed at most remote. The deaths of two prominent herpetologists, Robert Mertens and Karl Schmidt, from African colubrid bites, changed that assessment, and recent events reveal that several other species of rear-fanged snakes have venoms that are potentially lethal to large vertebrates.
Boomslang (Dispholidus typus) and twig snake (Thelotornis spp.) venoms are toxic to blood cells and thin the blood (hemotoxic, hemorrhagic). Early symptoms include headaches, nausea, diarrhea, lethargy, mental disorientation, bruising, and bleeding at the site and all body openings. Exsanguination is the main cause of death from such a bite.
The boomslang's venom is the most potent of all rear-fanged snakes in the world based on LD50. Although its venom may be more potent than some vipers and elapids, it causes fewer fatalities owing to various factors (for example, the fangs' effectiveness is not high compared with many other snakes, the venom dose delivered is low, and boomslangs are generally less aggressive in comparison to other venomous snakes such as cobras and mambas). Symptoms of a bite from these snakes include nausea and internal bleeding, and one could die from a
Aglyphous snakes
Experiments made with the secretion of the
Use of snake venoms to treat disease
Given that snake venom contains many biologically active ingredients, some may be useful to treat disease.[42]
For instance,
The analgesic (pain-killing) activity of many snake venom proteins has been long known.[47][48] The main challenge, however, is how to deliver protein to the nerve cells: proteins usually are not applicable as pills.
Immunity
Among snakes
The question whether individual snakes are immune to their own venom has not yet been definitively settled, though an example is known of a cobra that self-envenomated, resulting in a large
Among other animals
The hedgehog (Erinaceidae), the mongoose (Herpestidae), the honey badger (Mellivora capensis) and the opossum are known to be immune to a dose of snake venom.[citation needed] Recently, the honey badger and domestic pig were found to have convergently evolved amino-acid replacements in their nicotinic acetylcholine receptor, which are known to confer resistance to alpha-neurotoxins in hedgehogs.[50] Whether the pig may be considered immune is still uncertain, though early studies show endogenous resistance in pigs tested against neurotoxins.[51] Though the pig's subcutaneous layer of fat may protect it against snake venom, most venoms pass easily through vascular fat layers, making this unlikely to contribute to its ability to resist venoms. The garden dormouse (Eliomys quercinus) has recently been added to the list of animals refractory to viper venom. Some populations of California ground squirrel (Otospermophilus beecheyi) are at least partially immune to rattlesnake venom as adults.
Among humans
The acquisition of human immunity against snake venom is ancient (from around 60 CE, Psylli tribe). Research into development of vaccines that will lead to immunity is ongoing. Bill Haast, owner and director of the Miami Serpentarium, injected himself with snake venom during most of his adult life, in an effort to build up an immunity to a broad array of venomous snakes, in a practice known as mithridatism. Haast lived to age 100, and survived a reported 172 snake bites. He donated his blood to be used in treating snake-bite patients when a suitable antivenom was not available. More than 20 so-treated individuals recovered.[52][53][54] Amateur researcher Tim Friede also lets venomous snakes bite him in the hopes of a vaccine against snake venom being developed, and has survived over 160 bites from different species as of January 2016.[55]
Traditional treatments
The World Health Organization estimates that 80% of the world's population depends on traditional medicine for their primary health-care needs.[56] Methods of traditional treatments of snakebites, although of questionable efficacy and perhaps even harmful, are nonetheless relevant.
Plants used to treat snakebites in Trinidad and Tobago are made into tinctures with alcohol or olive oil and kept in rum flasks called snake bottles, which contain several different plants and/or insects. The plants used include the vine called monkey ladder (
Serotherapy
Serotherapy using antivenom is a common current treatment and has been described back in 1913.[note 1] Both adaptive immunity and serotherapy are specific to the type of snake; venom with identical physiological action do not cross-neutralize. Boulenger 1913 describes the following cases:
A European in Australia who had become immune to the venom of the deadly Australian tiger snake (Notechis scutatus), manipulating these snakes with impunity, and was under the impression that his immunity extended also to other species, when bitten by a lowland copperhead (Austrelaps superbus), an allied elapine, died the following day.
In
In Brazil, serum prepared with the venom of lanceheads (Bothrops spp.) is without action on rattlesnake (Crotalus spp.) venom.
Antivenom snakebite treatment must be matched as the type of envenomation that has occurred. In the Americas, polyvalent antivenoms are available that are effective against the bites of most pit vipers.
Notes
- ^ This section is based on the 1913 book The Snakes of Europe, by G. A. Boulenger, which is now in the public domain in the United States (and possibly elsewhere) Because of its age, the text in this article should not necessarily be viewed as reflecting the current knowledge of snake venom
See also
References
- ^ "Reptile Venom Research". Australian Reptile Park. Archived from the original on 2 February 2010. Retrieved 21 December 2010.
- ^ ISBN 978-1-4027-3181-5.
- ^ ISBN 978-1-55297-613-5.
- ^ PMID 21544178.
- ^ PMID 35702592.
- ISBN 978-0-691-13295-2.
- PMID 20977763.
- PMID 14124757.

- S2CID 8952817.

- ISBN 978-0-429-05420-4.)
{{cite book}}: CS1 maint: location (link - ^ a b "Keyword: Hemorrhagic toxin KW-1200". UniProt. Retrieved 1 June 2019.
- S2CID 20158638.

- PMID 15302536.

- PMID 25313513.
- S2CID 205728095.

- S2CID 45019479.
- PMID 1862521.
- PMID 18463304.
- PMID 524395.
- .
- ^ PMID 11732525.
- PMID 1782484.
- ^ PMID 22446061.
- ^ PMID 23219381.
- PMID 24576642.
- PMID 25079342.
- PMID 17233905.

- PMID 19364745.
- PMID 17900344.

- ^ PMID 19364745.

- PMID 21731638.
- PMID 22643073.
- S2CID 17572816.
- PMID 20227433.
- PMID 23452837.
- S2CID 250188352.
- PMID 31817769.
- PMID 17671964.

- PMID 28768797.
- S2CID 233411378.
- ^ Martin CJ, Lamb G (1907). "Snake-poison and Snake-bite". In Allbutt TC, Rolleston ND (eds.). A System of Medicine. London: MacMillan. pp. 783–821.
- PMID 23058997.
- PMID 23509718.
- PMID 23593597.
- PMID 23244070.
- S2CID 15127065.
- S2CID 8697382.
- S2CID 36274766.
- ^ "Sterile tail abscess in Naja annulifera - self-envenomation case". Archived from the original on 27 October 2004. Retrieved 2 April 2009.
- PMID 25796346.
- .
- ^ "Farewell to these famous Floridians". Florida Trend. 19 December 2011. Retrieved 2 April 2012.
- ^ Rosenberg C (21 June 2011). "Bill Haast dies at 100; snakes were the charm for south Florida celebrity". Los Angeles Times. Retrieved 16 October 2012.
- ^ Schudel M (18 June 2011). "Bill Haast dies at 100: Florida snake man provided venom for snakebite serum". The Washington Post. Retrieved 16 October 2012.
- Independent.co.uk. 21 January 2016. Retrieved 7 July 2016.
- ^ Hiremath VT, Taranath TC (February 2010). "Traditional Phytotherapy for Snake bites by Tribes of Chitradurga District, Karnataka, India". Ethnobotanical Leaflets. 14 (2): 120–125.
- PMID 7057657.

- ^ "Treating Snake Bites". Ces.ncsu.edu. Retrieved 16 October 2012.
- ^ "CDC - Venomous Snakes - NIOSH Workplace Safety and Health Topic". CDC.gov. 1 July 2016. Retrieved 7 July 2016.
- ^ http://www.savagelabs.com/Products/CroFab/Home/crofab_frame.htm Archived 3 March 2016 at the Wayback Machine Link to PDF for full prescribing information, retrieved 11/12/12
Further reading
- Jonassen I, Collins JF, Higgins DG (August 1995). "Finding flexible patterns in unaligned protein sequences". Protein Science. 4 (8): 1587–95. PMID 8520485.
- Shaw IC (2007). "Chapter 19: Snake Toxins". In Waring RH, Steventon GB, Mitchell SC (eds.). Molecules of Death (Second ed.). River Edge, N.J: Imperial College Press. pp. 329–344. ISBN 978-1-86094-815-2.
External links
- An overview of the diversity and evolution of snake fangs.
- Snake VenomsUMich Orientation of Proteins in Membranes families/superfamily-55 - Calculated orientations of snake venom phospholipases A2 and myotoxins in the lipid bilayer.
- LD50's for most toxic venoms.
- Australian Venom Research Unit - a general source of information for venomous creatures in Australia.
- biomedcentral.com - Medicinal and ethnoveterinary remedies of hunters in Trinidad.
- reptilis.net - How venom works.
- snakevenom.net - Drying and storage of snake venom.