Soil
Soil, also commonly referred to as earth or . Some scientific definitions distinguish dirt from soil by restricting the former term specifically to displaced soil.

Soil consists of a solid phase of minerals and organic matter (the soil matrix), as well as a porous phase that holds gases (the soil atmosphere) and water (the soil solution).[1][2] Accordingly, soil is a three-state system of solids, liquids, and gases.[3] Soil is a product of several factors: the influence of climate, relief (elevation, orientation, and slope of terrain), organisms, and the soil's parent materials (original minerals) interacting over time.[4] It continually undergoes development by way of numerous physical, chemical and biological processes, which include weathering with associated erosion.[5] Given its complexity and strong internal connectedness, soil ecologists regard soil as an ecosystem.[6]
Most soils have a dry
Collectively the Earth's body of soil is called the pedosphere. The pedosphere interfaces with the lithosphere, the hydrosphere, the atmosphere, and the biosphere.[10] Soil has four important functions:
- as a medium for plant growth
- as a means of water storage, supply, and purification
- as a modifier of Earth's atmosphere
- as a habitat for organisms
All of these functions, in their turn, modify the soil and its properties.
Soil science has two basic branches of study: edaphology and pedology. Edaphology studies the influence of soils on living things.[11] Pedology focuses on the formation, description (morphology), and classification of soils in their natural environment.[12] In engineering terms, soil is included in the broader concept of regolith, which also includes other loose material that lies above the bedrock, as can be found on the Moon and other celestial objects.[13]
Processes
Soil is a major component of the
Soil acts as an engineering medium, a habitat for
Soils can effectively remove impurities,
Composition

Components of a silt loam soil by percent volume
A typical soil is about 50% solids (45% mineral and 5% organic matter), and 50% voids (or pores) of which half is occupied by water and half by gas.
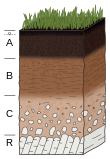
Given sufficient time, an undifferentiated soil will evolve a soil profile that consists of two or more layers, referred to as soil horizons. These differ in one or more properties such as in their texture, structure, density, porosity, consistency, temperature, color, and reactivity.[8] The horizons differ greatly in thickness and generally lack sharp boundaries; their development is dependent on the type of parent material, the processes that modify those parent materials, and the soil-forming factors that influence those processes. The biological influences on soil properties are strongest near the surface, though the geochemical influences on soil properties increase with depth. Mature soil profiles typically include three basic master horizons: A, B, and C. The solum normally includes the A and B horizons. The living component of the soil is largely confined to the solum, and is generally more prominent in the A horizon.[36] It has been suggested that the pedon, a column of soil extending vertically from the surface to the underlying parent material and large enough to show the characteristics of all its horizons, could be subdivided in the humipedon (the living part, where most soil organisms are dwelling, corresponding to the humus form), the copedon (in intermediary position, where most weathering of minerals takes place) and the lithopedon (in contact with the subsoil).[37]
The soil texture is determined by the relative proportions of the individual particles of
Water is a critical agent in soil development due to its involvement in the dissolution, precipitation, erosion, transport, and deposition of the materials of which a soil is composed.
Soils supply
Plant nutrient availability is affected by soil pH, which is a measure of the hydrogen ion activity in the soil solution. Soil pH is a function of many soil forming factors, and is generally lower (more acidic) where weathering is more advanced.[43]
Most plant nutrients, with the exception of
Formation
Soil is said to be formed when organic matter has accumulated and colloids are washed downward, leaving deposits of clay,
An example of the development of a soil would begin with the weathering of lava flow bedrock, which would produce the purely mineral-based parent material from which the soil texture forms. Soil development would proceed most rapidly from bare rock of recent flows in a warm climate, under heavy and frequent rainfall. Under such conditions, plants (in a first stage
How soil formation proceeds is influenced by at least five classic factors that are intertwined in the evolution of a soil: parent material, climate, topography (relief), organisms, and time.[56] When reordered to climate, relief, organisms, parent material, and time, they form the acronym CROPT.[57]
Physical properties
The physical properties of soils, in order of decreasing importance for ecosystem services such as
Soil moisture
Soil
Available water capacity is the amount of water held in a soil profile available to plants. As water content drops, plants have to work against increasing forces of adhesion and sorptivity to withdraw water. Irrigation scheduling avoids moisture stress by replenishing depleted water before stress is induced.[64][65]
Capillary action is responsible for moving groundwater from wet regions of the soil to dry areas. Subirrigation designs (e.g., wicking beds, sub-irrigated planters) rely on capillarity to supply water to plant roots. Capillary action can result in an evaporative concentration of salts, causing land degradation through salination.
Soil gas
The atmosphere of soil, or
Soil atmosphere is also the seat of emissions of volatiles other than carbon and nitrogen oxides from various soil organisms, e.g. roots,[76] bacteria,[77] fungi,[78] animals.[79] These volatiles are used as chemical cues, making soil atmosphere the seat of interaction networks[80][81] playing a decisive role in the stability, dynamics and evolution of soil ecosystems.[82] Biogenic soil volatile organic compounds are exchanged with the aboveground atmosphere, in which they are just 1–2 orders of magnitude lower than those from aboveground vegetation.[83]
Humans can get some idea of the soil atmosphere through the well-known 'after-the-rain' scent, when infiltering rainwater flushes out the whole soil atmosphere after a drought period, or when soil is excavated,.
Solid phase (soil matrix)
Soil particles can be classified by their chemical composition (mineralogy) as well as their size. The particle size distribution of a soil, its texture, determines many of the properties of that soil, in particular hydraulic conductivity and water potential,[85] but the mineralogy of those particles can strongly modify those properties. The mineralogy of the finest soil particles, clay, is especially important.[86]
Soil biodiversity
Large numbers of microbes, animals, plants and fungi are living in soil. However, biodiversity in soil is much harder to study as most of this life is invisible, hence estimates about soil biodiversity have been unsatisfactory. A recent study suggested that soil is likely home to 59 ± 15% of the species on Earth. Enchytraeidae (worms) have the greatest percentage of species in soil (98.6%), followed by fungi (90%), plants (85.5%), and termites (Isoptera) (84.2%). Many other groups of animals have substantial fractions of species living in soil, e.g. about 30% of insects, and close to 50% of arachnids.[87] While most vertebrates live above ground (ignoring aquatic species), many species are fossorial, that is, they live in soil, such as most blind snakes.
Chemistry
The chemistry of a soil determines its ability to supply available
Cation and anion exchange
The cation exchange, that takes place between colloids and soil water, buffers (moderates) soil pH, alters soil structure, and purifies percolating water by adsorbing cations of all types, both useful and harmful.
The negative or positive charges on colloid particles make them able to hold cations or anions, respectively, to their surfaces. The charges result from four sources.[90]
- Isomorphous substitution occurs in clay during its formation, when lower-valence cations substitute for higher-valence cations in the crystal structure.[91] Substitutions in the outermost layers are more effective than for the innermost layers, as the electric charge strength drops off as the square of the distance. The net result is oxygen atoms with net negative charge and the ability to attract cations.
- Edge-of-clay oxygen atoms are not in balance ionically as the tetrahedral and octahedral structures are incomplete.[92]
- Hydroxyls may substitute for oxygens of the silica layers, a process called hydroxylation. When the hydrogens of the clay hydroxyls are ionised into solution, they leave the oxygen with a negative charge (anionic clays).[93]
- Hydrogens of humus hydroxyl groups may also be ionised into solution, leaving, similarly to clay, an oxygen with a negative charge.[94]
Cations held to the negatively charged colloids resist being washed downward by water and are out of reach of plant roots, thereby preserving the soil fertility in areas of moderate rainfall and low temperatures.[95][96]
There is a hierarchy in the process of cation exchange on colloids, as cations differ in the strength of adsorption by the colloid and hence their ability to replace one another (ion exchange). If present in equal amounts in the soil water solution:
Al3+ replaces H+ replaces Ca2+ replaces Mg2+ replaces K+ same as NH+
4 replaces Na+[97]
If one cation is added in large amounts, it may replace the others by the sheer force of its numbers. This is called law of mass action. This is largely what occurs with the addition of cationic fertilisers (potash, lime).[98]
As the soil solution becomes more acidic (low pH, meaning an abundance of H+), the other cations more weakly bound to colloids are pushed into solution as hydrogen ions occupy exchange sites (protonation). A low pH may cause the hydrogen of hydroxyl groups to be pulled into solution, leaving charged sites on the colloid available to be occupied by other cations. This ionisation of hydroxy groups on the surface of soil colloids creates what is described as pH-dependent surface charges.[99] Unlike permanent charges developed by isomorphous substitution, pH-dependent charges are variable and increase with increasing pH.[100] Freed cations can be made available to plants but are also prone to be leached from the soil, possibly making the soil less fertile.[101] Plants are able to excrete H+ into the soil through the synthesis of organic acids and by that means, change the pH of the soil near the root and push cations off the colloids, thus making those available to the plant.[102]
Cation exchange capacity (CEC)
Most of the soil's CEC occurs on clay and humus colloids, and the lack of those in hot, humid, wet climates (such as tropical rainforests), due to leaching and decomposition, respectively, explains the apparent sterility of tropical soils.[105] Live plant roots also have some CEC, linked to their specific surface area.[106]
| Soil | State | CEC meq/100 g |
|---|---|---|
| Charlotte fine sand | Florida | 1.0 |
| Ruston fine sandy loam | Texas | 1.9 |
| Glouchester loam | New Jersey | 11.9 |
| Grundy silt loam | Illinois | 26.3 |
| Gleason clay loam | California | 31.6 |
| Susquehanna clay loam | Alabama | 34.3 |
| Davie mucky fine sand | Florida | 100.8 |
| Sands | — | 1–5 |
| Fine sandy loams | — | 5–10 |
| Loams and silt loams | — | 5–15 |
| Clay loams | — | 15–30 |
| Clays | — | over 30 |
| Sesquioxides | — | 0–3 |
| Kaolinite | — | 3–15 |
| Illite | — | 25–40 |
| Montmorillonite | — | 60–100 |
| Vermiculite (similar to illite) | — | 80–150 |
| Humus | — | 100–300 |
Anion exchange capacity (AEC)
Anion exchange capacity is the soil's ability to remove anions (such as nitrate, phosphate) from the soil water solution and sequester those for later exchange as the plant roots release carbonate anions to the soil water solution.[108] Those colloids which have low CEC tend to have some AEC. Amorphous and sesquioxide clays have the highest AEC,[109] followed by the iron oxides.[110] Levels of AEC are much lower than for CEC, because of the generally higher rate of positively (versus negatively) charged surfaces on soil colloids, to the exception of variable-charge soils.[111] Phosphates tend to be held at anion exchange sites.[112]
Iron and aluminum hydroxide clays are able to exchange their hydroxide anions (OH−) for other anions.[108] The order reflecting the strength of anion adhesion is as follows:
- H
2PO−
4 replaces SO2−
4 replaces NO−
3 replaces Cl−
The amount of exchangeable anions is of a magnitude of tenths to a few milliequivalents per 100 g dry soil.[107] As pH rises, there are relatively more hydroxyls, which will displace anions from the colloids and force them into solution and out of storage; hence AEC decreases with increasing pH (alkalinity).[113]
Reactivity (pH)
Soil reactivity is expressed in terms of pH and is a measure of the acidity or alkalinity of the soil. More precisely, it is a measure of hydronium concentration in an aqueous solution and ranges in values from 0 to 14 (acidic to basic) but practically speaking for soils, pH ranges from 3.5 to 9.5, as pH values beyond those extremes are toxic to life forms.[114]
At 25 °C an aqueous solution that has a pH of 3.5 has 10−3.5 moles H3O+ (hydronium ions) per litre of solution (and also 10−10.5 moles per litre OH−). A pH of 7, defined as neutral, has 10−7 moles of hydronium ions per litre of solution and also 10−7 moles of OH− per litre; since the two concentrations are equal, they are said to neutralise each other. A pH of 9.5 has 10−9.5 moles hydronium ions per litre of solution (and also 10−2.5 moles per litre OH−). A pH of 3.5 has one million times more hydronium ions per litre than a solution with pH of 9.5 (9.5 − 3.5 = 6 or 106) and is more acidic.[115]
The effect of pH on a soil is to remove from the soil or to make available certain ions. Soils with high acidity tend to have toxic amounts of
In high rainfall areas, soils tend to acidify as the basic cations are forced off the soil colloids by the mass action of hydronium ions from usual or unusual
Base saturation percentage
There are acid-forming cations (e.g. hydronium, aluminium, iron) and there are base-forming cations (e.g. calcium, magnesium, sodium). The fraction of the negatively-charged soil colloid exchange sites (CEC) that are occupied by base-forming cations is called
Buffering
The resistance of soil to change in pH, as a result of the addition of acid or basic material, is a measure of the buffering capacity of a soil and (for a particular soil type) increases as the CEC increases. Hence, pure sand has almost no buffering ability, though soils high in colloids (whether mineral or organic) have high
The addition of a small amount of highly basic aqueous ammonia to a soil will cause the ammonium to displace hydronium ions from the colloids, and the end product is water and colloidally fixed ammonium, but little permanent change overall in soil pH.
The addition of a small amount of lime, Ca(OH)2, will displace hydronium ions from the soil colloids, causing the fixation of calcium to colloids and the evolution of CO2 and water, with little permanent change in soil pH.
The above are examples of the buffering of soil pH. The general principal is that an increase in a particular cation in the soil water solution will cause that cation to be fixed to colloids (buffered) and a decrease in solution of that cation will cause it to be withdrawn from the colloid and moved into solution (buffered). The degree of buffering is often related to the CEC of the soil; the greater the CEC, the greater the buffering capacity of the soil.[136]
Redox
Soil chemical reactions involve some combination of proton and electron transfer. Oxidation occurs if there is a loss of electrons in the transfer process while reduction occurs if there is a gain of electrons. Reduction potential is measured in volts or millivolts. Soil microbial communities develop along electron transport chains, forming electrically conductive biofilms, and developing networks of bacterial nanowires.
Redox factors in soil development, where formation of
Nutrients
| Element | Symbol | Ion or molecule |
|---|---|---|
| Carbon | C | CO2 (mostly through leaves) |
| Hydrogen | H | H+, H2O (water) |
| Oxygen | O | O2−, OH−, CO2− 3, SO2− 4, CO2 |
| Phosphorus | P | H 2PO− 4, HPO2− 4 (phosphates) |
| Potassium | K | K+ |
| Nitrogen | N | NH+ 4, NO− 3 (ammonium, nitrate) |
| Sulfur | S | SO2− 4 |
| Calcium | Ca | Ca2+ |
| Iron | Fe | Fe2+, Fe3+ (ferrous, ferric) |
| Magnesium | Mg | Mg2+ |
| Boron | B | H3BO3, H 2BO− 3, B(OH)− 4 |
| Manganese | Mn | Mn2+ |
| Copper | Cu | Cu2+ |
| Zinc | Zn | Zn2+ |
| Molybdenum | Mo | MoO2− 4 (molybdate) |
| Chlorine | Cl | Cl− (chloride) |
Seventeen elements or nutrients are essential for plant growth and reproduction. They are
Plant uptake of nutrients can only proceed when they are present in a plant-available form. In most situations, nutrients are absorbed in an
The nutrients adsorbed onto the surfaces of clay colloids and soil organic matter provide a more accessible reservoir of many plant nutrients (e.g. K, Ca, Mg, P, Zn). As plants absorb the nutrients from the soil water, the soluble pool is replenished from the surface-bound pool. The decomposition of soil organic matter by microorganisms is another mechanism whereby the soluble pool of nutrients is replenished – this is important for the supply of plant-available N, S, P, and B from soil.[144]
Gram for gram, the capacity of
Soil organic matter
The organic material in soil is made up of
A few percent of the soil organic matter, with small
The main part of soil organic matter is a complex assemblage of small organic molecules, collectively called humus or
Most living things in soils, including plants, animals, bacteria, and fungi, are dependent on organic matter for nutrients and/or energy. Soils have organic compounds in varying degrees of decomposition which rate is dependent on temperature, soil moisture, and aeration. Bacteria and fungi feed on the raw organic matter, which are fed upon by
In
Humus
Humus refers to organic matter that has been decomposed by soil microflora and fauna to the point where it is resistant to further breakdown. Humus usually constitutes only five percent of the soil or less by volume, but it is an essential source of nutrients and adds important textural qualities crucial to
Humus formation is a process dependent on the amount of plant material added each year and the type of base soil. Both are affected by climate and the type of organisms present.
Climatological influence
The production, accumulation and degradation of organic matter are greatly dependent on climate. For example, when a thawing event occurs, the flux of soil gases with atmospheric gases is significantly influenced.[178] Temperature, soil moisture and topography are the major factors affecting the accumulation of organic matter in soils. Organic matter tends to accumulate under wet or cold conditions where decomposer activity is impeded by low temperature[179] or excess moisture which results in anaerobic conditions.[180] Conversely, excessive rain and high temperatures of tropical climates enables rapid decomposition of organic matter and leaching of plant nutrients. Forest ecosystems on these soils rely on efficient recycling of nutrients and plant matter by the living plant and microbial biomass to maintain their productivity, a process which is disturbed by human activities.[181] Excessive slope, in particular in the presence of cultivation for the sake of agriculture, may encourage the erosion of the top layer of soil which holds most of the raw organic material that would otherwise eventually become humus.[182]
Plant residue
Typical types and percentages of plant residue components
Horizons
A horizontal layer of the soil, whose physical features, composition and age are distinct from those above and beneath, is referred to as a soil horizon. The naming of a horizon is based on the type of material of which it is composed. Those materials reflect the duration of specific processes of soil formation. They are labelled using a shorthand notation of letters and numbers which describe the horizon in terms of its colour, size, texture, structure, consistency, root quantity, pH, voids, boundary characteristics and presence of nodules or concretions.
The exposure of parent material to favourable conditions produces mineral soils that are marginally suitable for plant growth, as is the case in eroded soils.
Classification
One of the first soil classification systems was developed by Russian scientist Vasily Dokuchaev around 1880.[199] It was modified a number of times by American and European researchers and was developed into the system commonly used until the 1960s. It was based on the idea that soils have a particular morphology based on the materials and factors that form them. In the 1960s, a different classification system began to emerge which focused on soil morphology instead of parental materials and soil-forming factors. Since then, it has undergone further modifications. The World Reference Base for Soil Resources[200] aims to establish an international reference base for soil classification.
Uses
Soil is used in agriculture, where it serves as the anchor and primary nutrient base for plants. The types of soil and available moisture determine the species of plants that can be cultivated. Agricultural soil science was the primeval domain of soil knowledge, long time before the advent of pedology in the 19th century. However, as demonstrated by aeroponics, aquaponics and hydroponics, soil material is not an absolute essential for agriculture, and soilless cropping systems have been claimed as the future of agriculture for an endless growing mankind.[201]
Soil material is also a critical component in mining, construction and landscape development industries.
Soil resources are critical to the environment, as well as to food and fibre production, producing 98.8% of food consumed by humans.
The biological component of soil is an extremely important carbon sink since about 57% of the biotic content is carbon. Even in deserts, cyanobacteria, lichens and mosses form biological soil crusts which capture and sequester a significant amount of carbon by photosynthesis. Poor farming and grazing methods have degraded soils and released much of this sequestered carbon to the atmosphere. Restoring the world's soils could offset the effect of increases in greenhouse gas emissions and slow global warming, while improving crop yields and reducing water needs.[209][210][211]
Organic soils, especially peat, serve as a significant fuel and
Soils filter and purify water and affect its chemistry. Rain water and pooled water from ponds, lakes and rivers percolate through the soil horizons and the upper
Degradation
Land degradation is a human-induced or natural process which impairs the capacity of land to function.[222] Soil degradation involves acidification, contamination, desertification, erosion or salination.[223]
Acidification
Soil acidification is beneficial in the case of
Contamination
Soil
Microfibres from synthetic textiles are another type of plastic soil contamination, 100% of agricultural soil samples from southwestern China contained plastic particles, 92% of which were microfibres. Sources of microfibres likely included string or twine, as well as irrigation water in which clothes had been washed.[229]
The application of biosolids from sewage sludge and compost can introduce microplastics to soils. This adds to the burden of microplastics from other sources (e.g. the atmosphere). Approximately half the sewage sludge in Europe and North America is applied to agricultural land. In Europe it has been estimated that for every million inhabitants 113 to 770 tonnes of microplastics are added to agricultural soils each year.[229]
Desertification

Desertification, an environmental process of ecosystem degradation in arid and semi-arid regions, is often caused by badly adapted human activities such as overgrazing or excess harvesting of firewood. It is a common misconception that drought causes desertification.[230] Droughts are common in arid and semiarid lands. Well-managed lands can recover from drought when the rains return. Soil management tools include maintaining soil nutrient and organic matter levels, reduced tillage and increased cover.[231] These practices help to control erosion and maintain productivity during periods when moisture is available. Continued land abuse during droughts, however, increases land degradation. Increased population and livestock pressure on marginal lands accelerates desertification.[232] It is now questioned whether present-day climate warming will favour or disfavour desertification, with contradictory reports about predicted rainfall trends associated with increased temperature, and strong discrepancies among regions, even in the same country.[233]
Erosion

A serious and long-running water erosion problem occurs in
Soil piping is a particular form of soil erosion that occurs below the soil surface.[237] It causes levee and dam failure, as well as sink hole formation. Turbulent flow removes soil starting at the mouth of the seep flow and the subsoil erosion advances up-gradient.[238] The term sand boil is used to describe the appearance of the discharging end of an active soil pipe.[239]
Salination
Reclamation
Soils which contain high levels of particular clays with high swelling properties, such as smectites, are often very fertile. For example, the smectite-rich paddy soils of Thailand's Central Plains are among the most productive in the world. However, the overuse of mineral nitrogen fertilizers and pesticides in irrigated intensive rice production has endangered these soils, forcing farmers to implement integrated practices based on Cost Reduction Operating Principles.[243]
Many farmers in tropical areas, however, struggle to retain organic matter and clay in the soils they work. In recent years, for example, productivity has declined and soil erosion has increased in the low-clay soils of northern Thailand, following the abandonment of
In 2008, three years after the initial trials, IWMI scientists conducted a survey among 250 farmers in northeast Thailand, half of whom had applied bentonite to their fields. The average improvement for those using the clay addition was 18% higher than for non-clay users. Using the clay had enabled some farmers to switch to growing vegetables, which need more fertile soil. This helped to increase their income. The researchers estimated that 200 farmers in northeast Thailand and 400 in Cambodia had adopted the use of clays, and that a further 20,000 farmers were introduced to the new technique.[247]
If the soil is too high in clay or salts (e.g.
Adding organic matter, like ramial chipped wood or compost, to soil which is depleted in nutrients and too high in sand will boost its quality and improve production.[249][250]
Special mention must be made of the use of
History of studies and research
The history of the study of soil is intimately tied to humans' urgent need to provide food for themselves and forage for their animals. Throughout history, civilizations have prospered or declined as a function of the availability and productivity of their soils.[253]
Studies of soil fertility
The Greek historian
Experiments into what made plants grow first led to the idea that the ash left behind when plant matter was burned was the essential element but overlooked the role of nitrogen, which is not left on the ground after combustion, a belief which prevailed until the 19th century.[261] In about 1635, the Flemish chemist Jan Baptist van Helmont thought he had proved water to be the essential element from his famous five years' experiment with a willow tree grown with only the addition of rainwater. His conclusion came from the fact that the increase in the plant's weight had apparently been produced only by the addition of water, with no reduction in the soil's weight.[262][263][264] John Woodward (d. 1728) experimented with various types of water ranging from clean to muddy and found muddy water the best, and so he concluded that earthy matter was the essential element. Others concluded it was humus in the soil that passed some essence to the growing plant. Still others held that the vital growth principal was something passed from dead plants or animals to the new plants. At the start of the 18th century, Jethro Tull demonstrated that it was beneficial to cultivate (stir) the soil, but his opinion that the stirring made the fine parts of soil available for plant absorption was erroneous.[263][265]
As chemistry developed, it was applied to the investigation of soil fertility. The French chemist Antoine Lavoisier showed in about 1778 that plants and animals must combust oxygen internally to live. He was able to deduce that most of the 165-pound (75 kg) weight of van Helmont's willow tree derived from air.[266] It was the French agriculturalist Jean-Baptiste Boussingault who by means of experimentation obtained evidence showing that the main sources of carbon, hydrogen and oxygen for plants were air and water, while nitrogen was taken from soil.[267] Justus von Liebig in his book Organic chemistry in its applications to agriculture and physiology (published 1840), asserted that the chemicals in plants must have come from the soil and air and that to maintain soil fertility, the used minerals must be replaced.[268] Liebig nevertheless believed the nitrogen was supplied from the air. The enrichment of soil with guano by the Incas was rediscovered in 1802, by Alexander von Humboldt. This led to its mining and that of Chilean nitrate and to its application to soil in the United States and Europe after 1840.[269]
The work of Liebig was a revolution for agriculture, and so other investigators started experimentation based on it. In England John Bennet Lawes and Joseph Henry Gilbert worked in the Rothamsted Experimental Station, founded by the former, and (re)discovered that plants took nitrogen from the soil, and that salts needed to be in an available state to be absorbed by plants. Their investigations also produced the superphosphate, consisting in the acid treatment of phosphate rock.[270] This led to the invention and use of salts of potassium (K) and nitrogen (N) as fertilizers. Ammonia generated by the production of coke was recovered and used as fertiliser.[271] Finally, the chemical basis of nutrients delivered to the soil in manure was understood and in the mid-19th century chemical fertilisers were applied. However, the dynamic interaction of soil and its life forms was still not understood.
In 1856, J. Thomas Way discovered that ammonia contained in fertilisers was transformed into nitrates,[272] and twenty years later Robert Warington proved that this transformation was done by living organisms.[273] In 1890 Sergei Winogradsky announced he had found the bacteria responsible for this transformation.[274]
It was known that certain legumes could take up nitrogen from the air and fix it to the soil but it took the development of bacteriology towards the end of the 19th century to lead to an understanding of the role played in nitrogen fixation by bacteria. The symbiosis of bacteria and leguminous roots, and the fixation of nitrogen by the bacteria, were simultaneously discovered by the German agronomist Hermann Hellriegel and the Dutch microbiologist Martinus Beijerinck.[270]
Crop rotation, mechanisation, chemical and natural fertilisers led to a doubling of wheat yields in western Europe between 1800 and 1900.[275]
Studies of soil formation
The scientists who studied the soil in connection with agricultural practices had considered it mainly as a static substrate. However, soil is the result of evolution from more ancient geological materials, under the action of biotic and abiotic processes. After studies of the improvement of the soil commenced, other researchers began to study soil genesis and as a result also soil types and classifications.
In 1860, while in Mississippi, Eugene W. Hilgard (1833–1916) studied the relationship between rock material, climate, vegetation, and the type of soils that were developed. He realised that the soils were dynamic, and considered the classification of soil types.[276] His work was not continued. At about the same time, Friedrich Albert Fallou was describing soil profiles and relating soil characteristics to their formation as part of his professional work evaluating forest and farm land for the principality of Saxony. His 1857 book, Anfangsgründe der Bodenkunde (First principles of soil science), established modern soil science.[277] Contemporary with Fallou's work, and driven by the same need to accurately assess land for equitable taxation, Vasily Dokuchaev led a team of soil scientists in Russia who conducted an extensive survey of soils, observing that similar basic rocks, climate and vegetation types lead to similar soil layering and types, and established the concepts for soil classifications. Due to language barriers, the work of this team was not communicated to western Europe until 1914 through a publication in German by Konstantin Glinka, a member of the Russian team.[278]
Curtis F. Marbut, influenced by the work of the Russian team, translated Glinka's publication into English,[279] and, as he was placed in charge of the U.S. National Cooperative Soil Survey, applied it to a national soil classification system.[263]
See also
- Acid sulfate soil
- Agrophysics
- Crust
- Agricultural science
- Factors affecting permeability of soils
- Index of soil-related articles
- Mycorrhizal fungi and soil carbon storage
- Shrink–swell capacity
- Soil biodiversity
- Soil liquefaction
- Soil moisture velocity equation
- Soil zoology
- Tillage erosion
- World Soil Museum
- Red soil
References
- ISBN 978-0-12-546807-7. Archived(PDF) from the original on 10 July 2018. Retrieved 27 March 2022.
- ISBN 978-0-7167-0818-6.
- ISBN 9781292039398. Archived from the originalon 16 October 2022. Retrieved 27 March 2022.
- ISBN 978-0-7167-0269-6.
- ]
- S2CID 18251180. Retrieved 3 April 2022.
- ^ Yu, Charley; Kamboj, Sunita; Wang, Cheng; Cheng, Jing-Jy (2015). "Data collection handbook to support modeling impacts of radioactive material in soil and building structures" (PDF). Argonne National Laboratory. pp. 13–21. Archived (PDF) from the original on 4 August 2018. Retrieved 3 April 2022.
- ^ ISBN 978-0-470-96060-8. Archived from the originalon 22 April 2023. Retrieved 3 April 2022.
- (PDF) from the original on 13 November 2018. Retrieved 3 April 2022.
- ISBN 978-1-4020-3994-2. Archived(PDF) from the original on 5 September 2018. Retrieved 27 March 2022.
- ^ "Glossary of terms in soil science". Agriculture and Agri-Food Canada. 13 December 2013. Archived from the original on 27 October 2018. Retrieved 3 April 2022.
- CiteSeerX 10.1.1.552.237. Archived from the original(PDF) on 12 June 2018.
- ^ Küppers, Michael; Vincent, Jean-Baptiste. "Impacts and formation of regolith". Max Planck Institute for Solar System Research. Archived from the original on 4 August 2018. Retrieved 3 April 2022.
- PMID 33110065. Retrieved 3 April 2022.
- PMID 11833898. Retrieved 3 April 2022.
Our analysis of pedon data from several disturbed soil profiles suggests that physical disturbances and anthropogenic inputs of various materials (direct effects) can greatly alter the amount of C stored in these human "made" soils.
- S2CID 4404915. Retrieved 3 April 2022.
- S2CID 35007042. Archived from the originalon 22 September 2022. Retrieved 3 April 2022.
- S2CID 43955196. Archived from the original(PDF) on 10 April 2017. Retrieved 3 April 2022.
- (PDF) from the original on 8 August 2017. Retrieved 10 April 2022.
- S2CID 17779069. Retrieved 10 April 2022.
- PMID 12057676. Retrieved 10 April 2022.
- PMID 24489873.
- PMID 9618454.
- S2CID 94252768. Retrieved 10 April 2022.
- . Retrieved 10 April 2022.
- . Retrieved 10 April 2022.
- . Retrieved 10 April 2022.
- ^ "Community guide to monitored natural attenuation" (PDF). Retrieved 10 April 2022.
- doi:10.2136/sssaj1984.03615995004800060013x. Archived from the originalon 18 March 2023. Retrieved 10 April 2022.
- ISBN 9781405197700. Archived from the originalon 22 April 2023. Retrieved 10 April 2022.
- ISBN 978-92-5-105366-9. Retrieved 10 April 2022.
- ^ McClellan, Tai. "Soil composition". University of Hawaiʻi at Mānoa, College of Tropical Agriculture and Human Resources. Retrieved 18 April 2022.
- ^ "Arizona Master Gardener Manual". Cooperative Extension, College of Agriculture, University of Arizona. 9 November 2017. Archived from the original on 29 May 2016. Retrieved 17 December 2017.
- ^ S2CID 297400. Retrieved 18 April 2022.
- . Retrieved 18 April 2022.
- ^ Simonson 1957, p. 17.
- S2CID 197555747. Retrieved 18 April 2022.
- . Retrieved 18 April 2022.
- Food and Agriculture Organization of the United Nations. Retrieved 18 April 2022.
- . Retrieved 18 April 2022.
- ISBN 978-0132279383. Retrieved 18 April 2022.
- ^ "Soil colloids: properties, nature, types and significance" (PDF). Tamil Nadu Agricultural University. Retrieved 18 April 2022.
- ^ Miller, Jarrod O. "Soil pH affects nutrient availability". Retrieved 18 April 2022.
- .
- ISBN 978-1-4831-8568-2. Archived from the originalon 22 March 2023. Retrieved 24 April 2022.
- . Retrieved 24 April 2022.
- .
- .
- S2CID 18220447. Retrieved 24 April 2022.
- PMID 24463576.
- S2CID 128498195. Archived from the originalon 27 March 2022. Retrieved 24 April 2022.
- PMID 16918551.
- doi:10.1016/S0169-555X(02)00143-5. Archived from the originalon 22 April 2023. Retrieved 24 April 2022.
- . Retrieved 21 March 2021.
- . Retrieved 24 April 2022.
- McGraw-Hill. Archived(PDF) from the original on 8 August 2017. Retrieved 24 April 2022.
- ^ Ritter, Michael E. "The physical environment: an introduction to physical geography" (PDF). Retrieved 24 April 2022.
- Food and Agriculture Organization of the United Nations. Archived from the original(PDF) on 8 August 2017.
- . Retrieved 7 August 2022.
- (PDF) from the original on 16 May 2022. Retrieved 26 October 2023.
- . Retrieved 7 August 2022.
- ^ Tamboli, Prabhakar Mahadeo (1961). The influence of bulk density and aggregate size on soil moisture retention. Ames, Iowa: Iowa State University. Retrieved 7 August 2022.
- ^ S2CID 25639544.
- ^ "Water holding capacity". Oregon State University. 24 June 2016. Retrieved 9 October 2022.
Irrigators must have knowledge of the readily available moisture capacity so that water can be applied before plants have to expend excessive energy to extract moisture
- University of Minnesota Extension. Retrieved 9 October 2022.
Only a portion of the available water holding capacity is easily used by the crop before crop water stress develop
- PMID 33874575.
- S2CID 6161016. Retrieved 13 November 2022.
- PMID 16653979.
- PMID 12651534.
- . Retrieved 13 November 2022.
- ^ S2CID 18442559. Retrieved 13 November 2022.
- ^ Russell 1957, pp. 35–36.
- .
- S2CID 25691034. Retrieved 13 November 2022.
- . Retrieved 13 November 2022.
- S2CID 30214059. Retrieved 13 November 2022.
- PMID 12684534.
- S2CID 14509047. Retrieved 13 November 2022.
- ^ Purrington, Foster Forbes; Kendall, Paricia A.; Bater, John E.; Stinner, Benjamin R. (1991). "Alarm pheromone in a gregarious poduromorph collembolan (Collembola: Hypogastruridae)". Great Lakes Entomologist. 24 (2): 75–78. Retrieved 13 November 2022.
- PMID 19875278. Retrieved 13 November 2022.
- S2CID 26647480. Retrieved 13 November 2022.
- S2CID 6840457. Retrieved 13 November 2022.
- PMID 24689847.
- ^ Buzuleciu, Samuel A.; Crane, Derek P.; Parker, Scott L. (2016). "Scent of disinterred soil as an olfactory cue used by raccoons to locate nests of diamond-backed terrapins (Malaclemys terrapin)" (PDF). Herpetological Conservation and Biology. 11 (3): 539–551. Retrieved 27 November 2022.
- (PDF) from the original on 2 September 2018. Retrieved 15 January 2023.
- ^ College of Tropical Agriculture and Human Resources. "Soil mineralogy". University of Hawaiʻi at Mānoa. Retrieved 15 January 2023.
- PMID 37549278.
- ^ Sposito, Garrison (1984). The surface chemistry of soils. New York: Oxford University Press. Retrieved 15 January 2023.
- ^ Wynot, Christopher. "Theory of diffusion in colloidal suspensions". Retrieved 15 January 2023.
- ^ Donahue, Miller & Shickluna 1977, p. 103–106.
- PMID 10097044.
- S2CID 97428106. Retrieved 15 January 2023.
- S2CID 97507578. Retrieved 15 January 2023.
- S2CID 94333872. Retrieved 15 January 2023.
- ^ Pettit, Robert E. "Organic matter, humus, humate, humic acid, fulvic acid and humin: their importance in soil fertility and plant health" (PDF). Retrieved 15 January 2023.
- ^ Diamond, Sidney; Kinter, Earl B. (1965). "Mechanisms of soil-lime stabilization: an interpretive review" (PDF). Highway Research Record. 92: 83–102. Retrieved 15 January 2023.
- . Retrieved 15 January 2023.
- .
- S2CID 12462516. Retrieved 15 January 2023.
- ^ Chakraborty, Meghna (8 August 2022). "What is cation exchange capacity in soils?". Retrieved 15 January 2023.
- PMID 21090742. Retrieved 15 January 2023.
- (PDF) from the original on 19 August 2019. Retrieved 15 January 2023.
- ]
- ^ Donahue, Miller & Shickluna 1977, p. 114.
- S2CID 4301023. Retrieved 29 January 2023.
- ]
- ^ a b Donahue, Miller & Shickluna 1977, pp. 115–116.
- ^ S2CID 8562338. Retrieved 29 January 2023.
- )
- . Retrieved 29 January 2023.
- S2CID 4505438. Retrieved 29 January 2023.
- .
- S2CID 52972476. Retrieved 29 January 2023.
- ^ Robertson, Bryan. "pH requirements of freshwater aquatic life" (PDF). Archived from the original (PDF) on 8 May 2021. Retrieved 6 June 2021.
- )
- S2CID 97507578.
- ISBN 978-1845939953. Retrieved 13 June 2021.
- ^ Donahue, Miller & Shickluna 1977, pp. 116–117.
- . Retrieved 13 June 2021.
- PMID 28847136. Retrieved 13 June 2021.
- S2CID 44160220. Retrieved 13 June 2021.
- .
- . Retrieved 13 June 2021.
- . Retrieved 13 June 2021.
- PMID 16510516.
- PMID 16653803.
- PMID 16658143.
- ^ Donahue, Miller & Shickluna 1977, pp. 116–119.
- ^ Ahmad, Sagheer; Ghafoor, Abdul; Qadir, Manzoor; Aziz, M. Abbas (2006). "Amelioration of a calcareous saline-sodic soil by gypsum application and different crop rotations". International Journal of Agriculture and Biology. 8 (2): 142–46. Retrieved 13 June 2021.
- .
- . Retrieved 20 June 2021.
- ^ Donahue, Miller & Shickluna 1977, pp. 119–120.
- PMID 10097044.
- ^ Sparks, Donald L. "Acidic and basic soils: buffering" (PDF). Davis, California: University of California, Davis, Department of Land, Air, and Water Resources. Retrieved 20 June 2021.
- ISBN 978-94-009-6985-8. Retrieved 21 June 2021.
- ^ Donahue, Miller & Shickluna 1977, pp. 120–121.
- ^ Donahue, Miller & Shickluna 1977, p. 125.
- ^ Dean 1957, p. 80.
- ^ Russel 1957, pp. 123–125.
- ^ ISBN 978-0133254488. Retrieved 10 December 2023.
- .
- PMID 14757584.
- ^ Dean 1957, pp. 80–81.
- ^ ISBN 978-92-5-105490-1. Retrieved 17 December 2023.
- . Retrieved 17 December 2023.
- . Retrieved 17 December 2023.
- ^ Donahue, Miller & Shickluna 1977, pp. 123–131.
- (PDF) from the original on 13 December 2016. Retrieved 4 July 2021.
- . Retrieved 4 July 2021.
- . Retrieved 4 July 2021.
- S2CID 45102095.
- S2CID 93834812.
- S2CID 205246638. Retrieved 4 July 2021.
- ^ ISBN 9780120007936. Retrieved 4 July 2021.
- . Retrieved 4 July 2021.
- ^ ISBN 978-0471522799. Retrieved 4 July 2021.
- ^ from the original on 29 January 2016.
- ^ Pettit, Robert E. "Organic matter, humus, humate, humic acid, fulvic acid and humin: their importance in soil fertility and plant health" (PDF). Retrieved 11 July 2021.
- .
- ISBN 978-3-642-78356-2. Retrieved 11 July 2021.
- ^ ISBN 978-0-444-81516-3. Retrieved 11 July 2021.
- S2CID 45102095. Retrieved 11 July 2021.
- . Retrieved 11 July 2021.
- PMID 23179622. Retrieved 11 July 2021.
- S2CID 4193782. Retrieved 11 July 2021.
- S2CID 54213404. Archived from the original(PDF) on 8 May 2021. Retrieved 18 July 2021.
- ^ Manjaiah, K.M.; Kumar, Sarvendra; Sachdev, M. S.; Sachdev, P.; Datta, S. C. (2010). "Study of clay–organic complexes". Current Science. 98 (7): 915–921. Retrieved 18 July 2021.
- S2CID 98176725.
- S2CID 6930871. Retrieved 18 July 2021.
- . Retrieved 18 July 2021.
- . Retrieved 18 July 2021.
- PMID 18811620.
- . Retrieved 18 July 2021.
- .
- S2CID 73640995. Retrieved 18 July 2021.
- . Retrieved 18 July 2021.
- PMID 24614647.
- . Retrieved 3 October 2021.
- hdl:10261/82461. Retrieved 25 July 2021.
- S2CID 40927739. Retrieved 25 July 2021.
- S2CID 55212899. Retrieved 25 July 2021.
- . Retrieved 25 July 2021.
- ^ ISBN 978-0-8493-2802-2.
- ^ "Horizons". Soils of Canada. Archived from the original on 22 September 2019. Retrieved 1 August 2021.
- . Retrieved 1 August 2021.
- . Retrieved 1 August 2021.
- ^ Ugolini, Fiorenzo C.; Dahlgren, Randy A. (2002). "Soil development in volcanic ash" (PDF). Global Environmental Research. 6 (2): 69–81. Retrieved 1 August 2021.
- . Retrieved 1 August 2021.
- . Retrieved 1 August 2021.
- . Retrieved 1 August 2021.
- S2CID 131032169. Retrieved 1 August 2021.
- . Retrieved 1 August 2021.
- . Retrieved 1 August 2021.
- .
- . Retrieved 8 August 2021.
- .
- S2CID 94900982. Retrieved 8 August 2021.
- .
- ^ Dokuchaev, Vasily Vasilyevich (1967). "Russian Chernozem". Jerusalem, Israel: Israel Program for Scientific Translations. Retrieved 15 August 2021.
- ^ IUSS Working Group WRB (2022). "World Reference Base for Soil Resources, 4th edition". IUSS, Vienna.
- PMID 31396245.
- ISBN 978-0643109650.
- . Retrieved 15 August 2021.
- PMID 31400601.
- doi:10.1016/j.ecolind.2013.11.010. Archived from the original(PDF) on 14 August 2021. Retrieved 15 August 2021.
- PMID 11235891. Retrieved 15 August 2021.
- ISBN 978-92-79-15806-3. Retrieved 15 August 2021.
- PMID 16701446. Retrieved 15 August 2021.
- S2CID 14890013. Retrieved 22 August 2021.
- S2CID 8574723. Retrieved 22 August 2021.
- Renewable Energy World. Archivedfrom the original on 1 November 2012. Retrieved 22 August 2021.
- . Retrieved 22 August 2021.
- ^ "Peatlands and farming". Stoneleigh, United Kingdom: National Farmers' Union of England and Wales. 6 July 2020. Retrieved 22 August 2021.
- PMID 22768100.
- S2CID 4404915.
- S2CID 19647911.
- . Retrieved 22 August 2021.
- (PDF) from the original on 7 November 2017. Retrieved 22 August 2021.
- PMID 19232804. Retrieved 22 August 2021.
- PMID 19068820. Retrieved 22 August 2021.
- S2CID 129416733. Retrieved 22 August 2021.
- . Retrieved 29 August 2021.
- ^ Oldeman, L. Roel (1993). "Global extent of soil degradation". ISRIC Bi-Annual Report 1991–1992. Wageningen, The Netherlands: International Soil Reference and Information Centre(ISRIC). pp. 19–36. Retrieved 29 August 2021.
- ^ Sumner, Malcolm E.; Noble, Andrew D. (2003). "Soil acidification: the world story" (PDF). In Rengel, Zdenko (ed.). Handbook of soil acidity. New York, NY, USA: Marcel Dekker. pp. 1–28. Archived from the original (PDF) on 14 August 2021. Retrieved 29 August 2021.
- . Retrieved 5 September 2021.
- PMID 11409985. Retrieved 5 September 2021.
- . Retrieved 5 September 2021.
- PMID 29709848.
- ^ a b Environment, U. N. (21 October 2021). "Drowning in Plastics – Marine Litter and Plastic Waste Vital Graphics". UNEP - UN Environment Programme. Retrieved 23 March 2022.
- . Retrieved 5 September 2021.
- ISSN 2071-1050.
- S2CID 4411922. Retrieved 5 September 2021.
- . Retrieved 5 September 2021.
- S2CID 129355387. Archived from the original(PDF) on 18 August 2021. Retrieved 5 September 2021.
- ISSN 0341-8162. Retrieved 5 September 2021.
- hdl:10722/228184. Retrieved 5 September 2021.
- . Retrieved 5 September 2021.
- . Retrieved 5 September 2021.
- ^ Dooley, Alan (June 2006). "Sandboils 101: Corps has experience dealing with common flood danger". Engineer Update. US Army Corps of Engineers. Archived from the original on 18 April 2008.
- ^ Oosterbaan, Roland J. (1988). "Effectiveness and social/environmental impacts of irrigation projects: a critical review" (PDF). Annual Reports of the International Institute for Land Reclamation and Improvement (ILRI). Wageningen, The Netherlands. pp. 18–34. Archived (PDF) from the original on 19 February 2009. Retrieved 5 September 2021.
- ISBN 978-0-16-061623-5. Retrieved 5 September 2021.
- ^ Oosterbaan, Roland J. "Waterlogging, soil salinity, field irrigation, plant growth, subsurface drainage, groundwater modelling, surface runoff, land reclamation, and other crop production and water management aspects". Archived from the original on 16 August 2010. Retrieved 5 September 2021.
- . Retrieved 12 September 2021.
- . Retrieved 12 September 2021.
- S2CID 18049595. Retrieved 12 September 2021.
- . Retrieved 12 September 2021.
- (PDF) from the original on 7 June 2012. Retrieved 12 September 2021.
- . Retrieved 12 September 2021.
- ^ Lemieux, Gilles; Germain, Diane (December 2000). "Ramial chipped wood: the clue to a sustainable fertile soil" (PDF). Université Laval, Département des Sciences du Bois et de la Forêt, Québec, Canada. Archived from the original (PDF) on 28 September 2021. Retrieved 12 September 2021.
- S2CID 96896374. Retrieved 12 September 2021.
- S2CID 26608101. Retrieved 12 September 2021.
- S2CID 52168678. Retrieved 12 September 2021.
- ISBN 978-0-520-08080-5.
- ^ a b Donahue, Miller & Shickluna 1977, p. 4.
- ^ Columella, Lucius Junius Moderatus (1745). Of husbandry, in twelve books, and his book concerning trees, with several illustrations from Pliny, Cato, Varro, Palladius, and other antient and modern authors, translated into English. London, United Kingdom: Andrew Millar. Retrieved 19 September 2021.
- ^ Kellogg 1957, p. 1.
- ^ Ibn al-'Awwam (1864). Le livre de l'agriculture, traduit de l'arabe par Jean Jacques Clément-Mullet. Filāḥah.French. (in French). Paris, France: Librairie A. Franck. Retrieved 19 September 2021.
- ISBN 978-0-87835-131-2.
- ^ de Serres, Olivier (1600). Le Théâtre d'Agriculture et mesnage des champs (in French). Paris, France: Jamet Métayer. Retrieved 19 September 2021.
- .
- PMID 12805882.
- ^ "Van Helmont's experiments on plant growth". BBC World Service. Retrieved 19 September 2021.
- ^ ISBN 978-0-02-313340-4. Retrieved 19 September 2021.
- ^ Kellogg 1957, p. 3.
- ^ Kellogg 1957, p. 2.
- ^ de Lavoisier, Antoine-Laurent (1777). "Mémoire sur la combustion en général" (PDF). Mémoires de l'Académie Royale des Sciences (in French). Retrieved 19 September 2021.
- ^ Boussingault, Jean-Baptiste (1860–1874). Agronomie, chimie agricole et physiologie, volumes 1–5 (in French). Paris, France: Mallet-Bachelier. Retrieved 19 September 2021.
- ^ von Liebig, Justus (1840). Organic chemistry in its applications to agriculture and physiology. London: Taylor and Walton. Retrieved 19 September 2021.
- ^ Way, J. Thomas (1849). "On the composition and money value of the different varieties of guano". Journal of the Royal Agricultural Society of England. 10: 196–230. Retrieved 19 September 2021.
- ^ a b Kellogg 1957, p. 4.
- ^ Tandon, Hari L.S. "A short history of fertilisers". Fertiliser Development and Consultation Organisation. Archived from the original on 23 January 2017. Retrieved 17 December 2017.
- ^ Way, J. Thomas (1852). "On the power of soils to absorb manure". Journal of the Royal Agricultural Society of England. 13: 123–143. Retrieved 19 September 2021.
- ^ Warington, Robert (1878). Note on the appearance of nitrous acid during the evaporation of water: a report of experiments made in the Rothamsted laboratory. London, United Kingdom: Harrison and Sons. Retrieved 19 September 2021.
- Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences(in French). 110 (1): 1013–1016. Retrieved 19 September 2021.
- ^ Kellogg 1957, pp. 1–4.
- The Macmillan Company. Retrieved 19 September 2021.
- ^ Fallou, Friedrich Albert (1857). Anfangsgründe der Bodenkunde (PDF) (in German). Dresden, Germany: G. Schönfeld's Buchhandlung. Archived from the original (PDF) on 15 December 2018. Retrieved 15 December 2018.
- Borntraeger.
- ^ Glinka, Konstantin Dmitrievich (1927). The great soil groups of the world and their development. Ann Arbor, Michigan: Edwards Brothers. Retrieved 19 September 2021.
Sources
![]() This article incorporates text from a free content work. Licensed under Cc BY-SA 3.0 IGO (license statement/permission). Text taken from Drowning in Plastics – Marine Litter and Plastic Waste Vital Graphics, United Nations Environment Programme.
This article incorporates text from a free content work. Licensed under Cc BY-SA 3.0 IGO (license statement/permission). Text taken from Drowning in Plastics – Marine Litter and Plastic Waste Vital Graphics, United Nations Environment Programme.
Bibliography
- Donahue, Roy Luther; Miller, Raymond W.; Shickluna, John C. (1977). Soils: An Introduction to Soils and Plant Growth. ISBN 978-0-13-821918-5.
- "Arizona Master Gardener". Cooperative Extension, College of Agriculture, University of Arizona. Retrieved 27 May 2013.
- Stefferud, Alfred, ed. (1957). Soil: The Yearbook of Agriculture 1957. United States Department of Agriculture. OCLC 704186906.
- Kellogg. "We Seek; We Learn". In Stefferud (1957).
- Simonson. "What Soils Are". In Stefferud (1957).
- Russell. "Physical Properties". In Stefferud (1957).
- Dean. "Plant Nutrition and Soil Fertility". In Stefferud (1957).
- Russel. "Boron and Soil Fertility". In Stefferud (1957).
Further reading
- Soil-Net.com Archived 10 July 2008 at the Wayback Machine A free schools-age educational site teaching about soil and its importance.
- Adams, J.A. 1986. Dirt. College Station, Texas: Texas A&M University Press ISBN 0-89096-301-0
- Certini, G., Scalenghe, R. 2006. Soils: Basic concepts and future challenges. Cambridge Univ Press, Cambridge.
- ISBN 978-0-520-25806-8
- Faulkner, Edward H. Plowman's Folly (New York, Grosset & Dunlap, 1943). ISBN 0-933280-51-3
- LandIS Free Soilscapes Viewer Free interactive viewer for the Soils of England and Wales
- Jenny, Hans. 1941. Factors of Soil Formation: A System of Quantitative Pedology
- Logan, W.B. Dirt: The ecstatic skin of the earth (1995). ISBN 1-57322-004-3
- Mann, Charles C. September 2008. " Our good earth" National Geographic Magazine
External links
- "97 Flood". USGS. Archived from the original on 24 June 2008. Retrieved 8 July 2008. Photographs of sand boils.
- Soil Survey Division Staff. 1999. Soil survey manual. Soil Conservation Service. U.S. Department of Agriculture Handbook 18.
- Soil Survey Staff. 1975. Soil Taxonomy: A basic system of soil classification for making and interpreting soil surveys. USDA-SCS Agric. Handb. 436. United States Government Printing Office, Washington, DC.
- Soils (Matching suitable forage species to soil type), Oregon State University
- Gardiner, Duane T. "Lecture 1 Chapter 1 Why Study Soils?". ENV320: Soil Science Lecture Notes. Texas A&M University-Kingsville. Archived from the original on 9 February 2018. Retrieved 7 January 2019.
- Janick, Jules. 2002. Soil notes, Purdue University
- LandIS Soils Data for England and Wales Archived 16 July 2007 at the Wayback Machine a pay source for GIS data on the soils of England and Wales and soils data source; they charge a handling fee to researchers.
- Short video explaining soil basics
- The Soil Water Compendium (soil water content sensors explained)
- Global Soil Partnership
- FAO Soils Portal
- World Reference Base for Soil Resources
- ISRIC – World Soil Information (ICSU World Data Centre for Soils)
- World Soil Library and Maps
- Wossac the world soil survey archive and catalogue
- Canadian Society of Soil Science
- Soil Science Society of America
- USDA-NRCS Web Soil Survey
- European Soil Portal (wiki)
- National Soil Resources Institute UK
- Plant and Soil Sciences eLibrary
- Copies of the reference 'Soil: The Yearbook of Agriculture 1957' in multiple formats