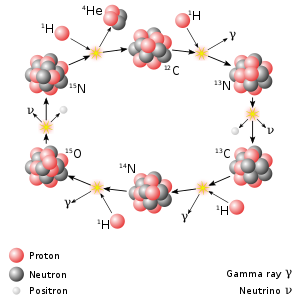Stellar nucleosynthesis
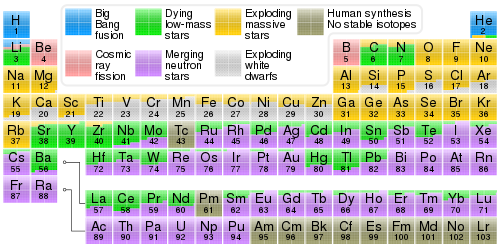
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a predictive theory, it yields accurate estimates of the observed abundances of the elements. It explains why the observed abundances of elements change over time and why some elements and their isotopes are much more abundant than others. The theory was initially proposed by Fred Hoyle in 1946,[1] who later refined it in 1954.[2] Further advances were made, especially to nucleosynthesis by neutron capture of the elements heavier than iron, by Margaret and Geoffrey Burbidge, William Alfred Fowler and Fred Hoyle in their famous 1957 B2FH paper,[3] which became one of the most heavily cited papers in astrophysics history.
The advanced sequence of burning fuels is driven by
A stimulus to the development of the theory of nucleosynthesis was the discovery of variations in the
History

In 1920,
In 1939, in a
Hoyle's theory was extended to other processes, beginning with the publication of the 1957 review paper "Synthesis of the Elements in Stars" by Burbidge, Burbidge, Fowler and Hoyle, more commonly referred to as the B2FH paper.[3] This review paper collected and refined earlier research into a heavily cited picture that gave promise of accounting for the observed relative abundances of the elements; but it did not itself enlarge Hoyle's 1954 picture for the origin of primary nuclei as much as many assumed, except in the understanding of nucleosynthesis of those elements heavier than iron by neutron capture. Significant improvements were made by Alastair G. W. Cameron and by Donald D. Clayton. In 1957 Cameron presented his own independent approach to nucleosynthesis,[14] informed by Hoyle's example, and introduced computers into time-dependent calculations of evolution of nuclear systems. Clayton calculated the first time-dependent models of the s-process in 1961[15] and of the r-process in 1965,[16] as well as of the burning of silicon into the abundant alpha-particle nuclei and iron-group elements in 1968,[17][18] and discovered radiogenic chronologies[19] for determining the age of the elements.

Key reactions
The most important reactions in stellar nucleosynthesis:
- Hydrogen fusion:
- Helium fusion:
- The triple-alpha process
- The alpha process
- Fusion of heavier elements:
- Lithium burning: a process found most commonly in brown dwarfs
- Carbon-burning process
- Neon-burning process
- Oxygen-burning process
- Silicon-burning process
- Production of elements heavier than iron:
- Neutron capture:
- Proton capture:
- The rp-process
- The p-process
- Photodisintegration
Hydrogen fusion
Hydrogen fusion (nuclear fusion of four protons to form a
In the cores of lower-mass main-sequence stars such as the
In higher-mass stars, the dominant energy production process is the
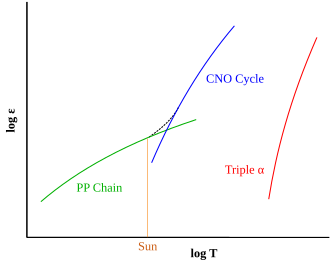
The type of hydrogen fusion process that dominates in a star is determined by the temperature dependency differences between the two reactions. The proton–proton chain reaction starts at temperatures about 4×106 K,[30] making it the dominant fusion mechanism in smaller stars. A self-maintaining CNO chain requires a higher temperature of approximately 1.6×107 K, but thereafter it increases more rapidly in efficiency as the temperature rises, than does the proton–proton reaction.[31] Above approximately 1.7×107 K, the CNO cycle becomes the dominant source of energy. This temperature is achieved in the cores of main-sequence stars with at least 1.3 times the mass of the Sun.[32] The Sun itself has a core temperature of about 1.57×107 K.[33]: 5 As a main-sequence star ages, the core temperature will rise, resulting in a steadily increasing contribution from its CNO cycle.[25]
Helium fusion
Main sequence stars accumulate helium in their cores as a result of hydrogen fusion, but the core does not become hot enough to initiate helium fusion. Helium fusion first begins when a star leaves the
In all cases, helium is fused to carbon via the triple-alpha process, i.e., three helium nuclei are transformed into carbon via 8Be.[35]: 30 This can then form oxygen, neon, and heavier elements via the alpha process. In this way, the alpha process preferentially produces elements with even numbers of protons by the capture of helium nuclei. Elements with odd numbers of protons are formed by other fusion pathways.[36]: 398
Reaction rate
The reaction rate density between species A and B, having number densities nA,B, is given by:
Semi-classically, the cross section is proportional to , where is the de Broglie wavelength. Thus semi-classically the cross section is proportional to .
However, since the reaction involves
One then integrates over all energies to get the total reaction rate, using the Maxwell–Boltzmann distribution and the relation:
Since this integration has an exponential damping at high energies of the form and at low energies from the Gamow factor, the integral almost vanished everywhere except around the peak, called Gamow peak,[37]: 185 at E0, where:
Thus:
The exponent can then be approximated around E0 as:
And the reaction rate is approximated as:[38]
Values of S(E0) are typically 10−3 – 103
Thus, the limiting reaction in the
References
Notes
- ^ Particle physicist Andrea Pocar points out, "Confirmation of CNO burning in our sun, where it operates at only one percent, reinforces our confidence that we understand how stars work."
- ^ "This result therefore paves the way toward a direct measurement of the solar metallicity using CNO neutrinos. Our findings quantify the relative contribution of CNO fusion in the Sun to be of the order of 1 per cent."—M. Agostini, et al.
Citations
- ^ a b Hoyle, F. (1946). "The synthesis of the elements from hydrogen". .
- ^ a b
Hoyle, F. (1954). "On Nuclear Reactions Occurring in Very Hot STARS. I. The Synthesis of Elements from Carbon to Nickel". doi:10.1086/190005.
- ^ a b Burbidge, E. M.; Burbidge, G. R.; Fowler, W.A.; Hoyle, F. (1957). "Synthesis of the Elements in Stars" (PDF). .
- ^ Suess, H. E.; Urey, H. C. (1956). "Abundances of the Elements". .
- ^ Clayton, D. D. (1968). Principles of Stellar Evolution and Nucleosynthesis. University of Chicago Press.
- ^
Eddington, A. S. (1920). "The internal constitution of the stars". PMID 17747682.
- ^
Eddington, A. S. (1920). "The Internal Constitution of the Stars". PMID 17747682.
- ^ Selle, D. (October 2012). "Why the Stars Shine" (PDF). Guidestar. Houston Astronomical Society. pp. 6–8. Archived (PDF) from the original on 2013-12-03.
- ^ Krane, K. S., Modern Physics (Hoboken, NJ: Wiley, 1983), p. 410.
- ^
Bethe, H. A. (1939). "Energy Production in Stars". PMID 17835673.
- ISBN 978-1-107-01638-5..
- ^ Clayton, D. D. (1968). Principles of Stellar Evolution and Nucleosynthesis. University of Chicago Press. p. 365.
- ^
Clayton, D. D. (2007). "History of Science: Hoyle's Equation". S2CID 118423007.
- ^ Cameron, A. G. W. (1957). Stellar Evolution, Nuclear Astrophysics, and Nucleogenesis (PDF) (Report). Atomic Energy of Canada Limited. Report CRL-41.
- ^ Clayton, D. D.; Fowler, W. A.; Hull, T. E.; Zimmerman, B. A. (1961). "Neutron capture chains in heavy element synthesis". .
- ^
Seeger, P. A.; Fowler, W. A.; Clayton, D. D. (1965). "Nucleosynthesis of Heavy Elements by Neutron Capture". doi:10.1086/190111.
- ^ Bodansky, D.; Clayton, D. D.; Fowler, W. A. (1968). "Nucleosynthesis During Silicon Burning". .
- ^
Bodansky, D.; Clayton, D. D.; Fowler, W. A. (1968). "Nuclear Quasi-Equilibrium during Silicon Burning". doi:10.1086/190176.
- ^
Clayton, D. D. (1964). "Cosmoradiogenic Chronologies of Nucleosynthesis". doi:10.1086/147791.
- ^ ISBN 978-0-313-34075-8
- Wadsworth Publishing Company, 1986), p. 245.
- ^ ISBN 978-0-521-34871-3
- S2CID 15159121.
- ^ ISBN 978-0-7923-1768-5
- ^ ISBN 978-3-642-10368-1
- ISBN 978-3-540-34143-7.
- ^ "Neutrinos yield first experimental evidence of catalyzed fusion dominant in many stars". phys.org. Retrieved 2020-11-26.
- ^ Choppin, G. R., Liljenzin, J.-O., Rydberg, J., & Ekberg, C., Radiochemistry and Nuclear Chemistry (Cambridge, MA: Academic Press, 2013), p. 357.
- S2CID 227174644.
- ISBN 978-3-540-25124-8.
- ISBN 978-0-470-09220-0
- S2CID 10626836
- ^ Wolf, E. L., Physics and Technology of Sustainable Energy (Oxford, Oxford University Press, 2018), p. 5.
- ^ Karttunen, H., Kröger, P., Oja, H., Poutanen, M., & Donner, K. J., eds., Fundamental Astronomy (Berlin/Heidelberg: Springer, 1987), p. 250.
- ^ Rehder, D., Chemistry in Space: From Interstellar Matter to the Origin of Life (Weinheim: Wiley-VCH, 2010), p. 30.
- ^ Perryman, M., The Exoplanet Handbook (Cambridge: Cambridge University Press, 2011), p. 398.
- ^ Iliadis, C., Nuclear Physics of Stars (Weinheim: Wiley-VCH, 2015), p. 185.
- ^ "University College London astrophysics course: lecture 7 – Stars" (PDF). Archived from the original (PDF) on January 15, 2017. Retrieved May 8, 2020.
- ^ Maoz, D., Astrophysics in a Nutshell (Princeton: Princeton University Press, 2007), ch. 3.
- S2CID 16061677.
- S2CID 119117147.
- ^ Goupil, M., Belkacem, K., Neiner, C., Lignières, F., & Green, J. J., eds., Studying Stellar Rotation and Convection: Theoretical Background and Seismic Diagnostics (Berlin/Heidelberg: Springer, 2013), p. 211.
Further reading
- Bethe, H. A. (1939). "Energy Production in Stars". PMID 17835673.
- Bethe, H. A. (1939). "Energy Production in Stars". PMID 17835673.
- Hoyle, F. (1954). "On Nuclear Reactions occurring in very hot stars: Synthesis of elements from carbon to nickel". doi:10.1086/190005.
- McGraw-Hill.
- Ray, A. (2004). "Stars as thermonuclear reactors: Their fuels and ashes". arXiv:astro-ph/0405568.
- hdl:2152/61093. Archived from the original(PDF) on 2009-03-26. Retrieved 2006-08-04.
- S2CID 55932331.
- Clayton, Donald D. (2003). Handbook of Isotopes in the Cosmos. Cambridge: ISBN 978-0-521-82381-4.
- Iliadis, Christian (2007). Nuclear Physics of Stars. Weinheim: Wiley-VCH. ISBN 978-3-527-40602-9.
External links
- "How the Sun Shines", by John N. Bahcall (Nobel prize site, accessed 6 January 2020)
- Nucleosynthesis in NASA's Cosmicopia