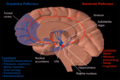
Substantia nigra
| Substantia nigra | |
|---|---|
Midbrain, basal ganglia | |
| Identifiers | |
| Latin | substantia nigra |
| Acronym(s) | SN |
| MeSH | D013378 |
| NeuroNames | 536 |
| NeuroLex ID | birnlex_789 |
| TA98 | A14.1.06.111 |
| TA2 | 5881 |
| FMA | 67947 |
| Anatomical terms of neuroanatomy] | |
The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons.[1] Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.[2]
Although the substantia nigra appears as a continuous band in brain sections, anatomical studies have found that it actually consists of two parts with very different connections and functions: the pars compacta (SNpc) and the pars reticulata (SNpr). The pars compacta serves mainly as a projection to the basal ganglia circuit, supplying the striatum with dopamine. The pars reticulata conveys signals from the basal ganglia to numerous other brain structures.[3]
Structure
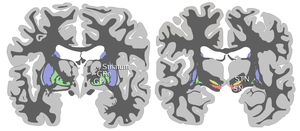

The substantia nigra, along with four other nuclei, is part of the
The SN is divided into two parts: the pars reticulata (SNpr) and the pars compacta (SNpc), which lies medial to the pars reticulata. Sometimes, a third region, the pars lateralis, is mentioned, though it is usually classified as part of the pars reticulata. The (SNpr) and the internal globus pallidus (GPi) are separated by the internal capsule.[4]
Pars reticulata
The pars reticulata bears a strong structural and functional resemblance to the internal part of the globus pallidus. The two are sometimes considered parts of the same structure, separated by the white matter of the internal capsule. Like those of the globus pallidus, the neurons in pars reticulata are mainly
Afferent connections
The main input to the SNpr derives from the
Efferent connections
Significant projections occur to the thalamus (ventral lateral and ventral anterior nuclei), superior colliculus, and other caudal nuclei from the pars reticulata (the nigrothalamic pathway),[6] which use GABA as their neurotransmitter. In addition, these neurons form up to five collaterals that branch within both the pars compacta and pars reticulata, likely modulating dopaminergic activity in the pars compacta.[7]
Function
The substantia nigra is an important player in brain function, in particular, in
Pars reticulata
The pars reticulata of the substantia nigra is an important processing center in the basal ganglia. The GABAergic neurons in the pars reticulata convey the final processed signals of the basal ganglia to the thalamus and superior colliculus. In addition, the pars reticulata also inhibits dopaminergic activity in the pars compacta via axon collaterals, although the functional organization of these connections remains unclear.
The GABAergic neurons of the pars reticulata spontaneously fire action potentials. In rats, the frequency of action potentials is roughly 25 Hz.[9] The purpose of these spontaneous action potentials is to inhibit targets of the basal ganglia, and decreases in inhibition are associated with movement.[10] The subthalamic nucleus gives excitatory input that modulates the rate of firing of these spontaneous action potentials. However, lesion of the subthalamic nucleus leads to only a 20% decrease in pars reticulata firing rate, suggesting that the generation of action potentials in the pars reticulata is largely autonomous,[11] as exemplified by the pars reticulata's role in saccadic eye movement. A group of GABAergic neurons from the pars reticulata projects to the superior colliculus, exhibiting a high level of sustained inhibitory activity.[12] Projections from the caudate nucleus to the superior colliculus also modulate saccadic eye movement. Altered patterns of pars reticulata firing such as single-spike or burst firing are found in Parkinson's disease[13] and epilepsy.[14]
Pars compacta
The most prominent function of the pars compacta is motor control,[15] though the substantia nigra's role in motor control is indirect; electrical stimulation of the substantia nigra does not result in movement, due to mediation of the striatum in the nigral influence of movement. The pars compacta sends excitatory input to the striatum via D1 pathway that excites and activates the striatum, resulting in the release of GABA onto the globus pallidus to inhibit its inhibitory effects on the thalamic nucleus. This causes the thalamocortical pathways to become excited and transmits motor neuron signals to the cerebral cortex to allow the initiation of movement, which is absent in Parkinson's disease. However, lack of pars compacta neurons has a large influence on movement, as evidenced by the symptoms of Parkinson's. The motor role of the pars compacta may involve fine motor control, as has been confirmed in animal models with lesions in that region.[16]
The pars compacta is heavily involved in learned responses to stimuli. In primates, dopaminergic neuron activity increases in the nigrostriatal pathway when a new stimulus is presented.[17] Dopaminergic activity decreases with repeated stimulus presentation.[17] However, behaviorally significant stimulus presentation (i.e. rewards) continues to activate dopaminergic neurons in the substantia nigra pars compacta. Dopaminergic projections from the ventral tegmental area (bottom part of the "midbrain" or mesencephalon) to the prefrontal cortex (mesocortical pathway) and to the nucleus accumbens (mesolimbic pathway – "meso" referring to "from the mesencephalon"... specifically the ventral tegmental area) are implicated in reward, pleasure, and addictive behavior. The pars compacta is also important in spatial learning, the observations about one's environment and location in space. Lesions in the pars compacta lead to learning deficits in repeating identical movements,[18] and some studies point to its involvement in a dorsal striatal-dependent, response-based memory system that functions relatively independent of the hippocampus, which is traditionally believed to subserve spatial or episodic-like memory functions.[19]
The pars compacta also plays a role in
Clinical significance
The substantia nigra is critical in the development of many diseases and syndromes, including parkinsonism and Parkinson's disease. There exist a study showing that high-frequency stimulation delivery to the left substantia nigra can induce transient acute depression symptoms.[23]
Parkinson's disease
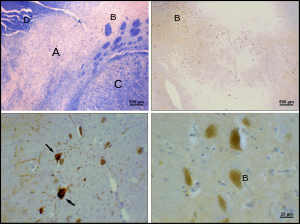
Parkinson's disease is a
The cause of death of dopaminergic neurons in the SNpc is unknown. However, some contributions to the unique susceptibility of dopaminergic neurons in the pars compacta have been identified. For one, dopaminergic neurons show abnormalities in mitochondrial complex 1, causing aggregation of alpha-synuclein; this can result in abnormal protein handling and neuron death.[26] Secondly, dopaminergic neurons in the pars compacta contain less calbindin than other dopaminergic neurons.[27] Calbindin is a protein involved in calcium ion transport within cells, and excess calcium in cells is toxic. The calbindin theory would explain the high cytotoxicity of Parkinson's in the substantia nigra compared to the ventral tegmental area. Regardless of the cause of neuronal death, the plasticity of the pars compacta is very robust; Parkinsonian symptoms do not generally appear until at least 30% of pars compacta dopaminergic neurons have died.[28] Most of this plasticity occurs at the neurochemical level; dopamine transport systems are slowed, allowing dopamine to linger for longer periods of time in the chemical synapses in the striatum.[29]
Menke, Jbabdi, Miller, Matthews and Zari (2010) used diffusion tensor imaging, as well as T1 mapping to assess volumetric differences in the SNpc and SNpr, in participants with Parkinson's compared to healthy individuals. These researchers found that participants with Parkinson's consistently had a smaller substantia nigra, specifically in the SNpr. Because the SNpr is connected to the posterior thalamus, ventral thalamus and specifically, the motor cortex, and because participants with Parkinson's disease report having a smaller SNprs (Menke, Jbabdi, Miller, Matthews and Zari, 2010), the small volume of this region may be responsible for motor impairments found in Parkinson's disease patients. This small volume may be responsible for weaker and/or less controlled motor movements, which may result in the tremors often experienced by those with Parkinson's.[30]
Oxidative stress and oxidative damage in the SNpc are likely key drivers in the etiology of Parkinson's disease as individuals age.[31] DNA damages caused by oxidative stress can be repaired by processes modulated by alpha-synuclein.[32] Alpha synuclein is expressed in the substantia nigra, but its DNA repair function appears to be compromised in Lewy body inclusion bearing neurons.[32] This loss may trigger cell death.
Schizophrenia
Increased levels of dopamine have long been implicated in the development of
Other, non-pharmacological evidence in support of the dopamine hypothesis relating to the substantia nigra include structural changes in the pars compacta, such as reduction in synaptic terminal size.
Wooden Chest Syndrome
Multiple system atrophy
Multiple system atrophy characterized by neuronal degeneration in the striatum and substantia nigra was previously called striatonigral degeneration.
Chemical modification of the substantia nigra
Chemical manipulation and modification of the substantia nigra is important in the fields of neuropharmacology and toxicology. Various compounds such as levodopa and MPTP are used in the treatment and study of Parkinson's disease, and many other drugs have effects on the substantia nigra.
Amphetamine and trace amines
Studies have shown that, in certain brain regions, amphetamine and trace amines increase the concentrations of dopamine in the
In addition, amphetamine and trace amines are substrates for the neuronal vesicular monoamine transporter, vesicular monoamine transporter 2 (VMAT2).[39] When amphetamine is taken up by VMAT2, the vesicle releases (effluxes) dopamine molecules into the cytosol in exchange.[39]
Cocaine
Inactivation of the substantia nigra could prove to be a possible treatment for cocaine addiction. In a study of cocaine-dependent rats, inactivation of the substantia nigra via implanted
Levodopa
The substantia nigra is the target of chemical therapeutics for the treatment of Parkinson's disease.
MPTP
Soon after, MPTP was tested in animal models for its efficacy in inducing Parkinson's disease (with success). MPTP induced akinesia, rigidity, and tremor in primates, and its neurotoxicity was found to be very specific to the substantia nigra pars compacta.
History
The substantia nigra was discovered in 1784 by Félix Vicq-d'Azyr,[52] and Samuel Thomas von Sömmerring alluded to this structure in 1791.[53] The differentiation between the substantia nigra pars reticulata and compacta was first proposed by Sano in 1910.[54] In 1963, Oleh Hornykiewicz concluded from his observation that "cell loss in the substantia nigra (of Parkinson's disease patients) could well be the cause of the dopamine deficit in the striatum."[55]
Additional images
-
Dopamine and serotonin
-
Degradation of substantia nigra associated with Parkinson's disease.
-
Horizontal MRI (T1 weighted) slice with highlighting indicating location of the substantia nigra.
-
Enhanced Neuromelanin MRI with Color images (RGB) showing Substantia nigra pars compacta
-
Microfilming
References
- S2CID 6769760.
- PMID 12917442.
- ISBN 978-0-08-045046-9, retrieved 7 September 2020
- ISBN 9780128022061.
- S2CID 43046462.
- S2CID 11790266.
- S2CID 20289802.
- PMID 10845063.
- S2CID 24485657.
- PMID 11896175.
- S2CID 10239473.
- PMID 6864250.
- S2CID 22886675.
- S2CID 6661257.
- S2CID 24642979.
- S2CID 11239586.
- ^ S2CID 18024404.
- PMID 17201473.
- S2CID 12045200.
- PMID 10649295.
- PMID 17551593.

- PMID 17035544.
- PMID 10320386.
- PMID 18344392.
- S2CID 19045599.
- S2CID 35486083.
- PMID 9117542.
- PMID 28066188.
- ^ Interview. Yoland Smith, PhD[verification needed]
- S2CID 19871414.
- PMID 31432604
- ^ PMID 31358782
- OCLC 458719.
- S2CID 2233617.
- PMID 16701550.
- S2CID 25752976.
- ^ PMID 21073468.
- ^ "Amphetamine". DrugBank. University of Alberta. 8 February 2013. Retrieved 13 October 2013.
- ^ PMID 21272013.
- PMID 19325074.
- PMID 575770.
- ISBN 978-0-8493-8813-2.
- PMID 2361170.
- ^ S2CID 34068876.
- S2CID 12652533.
- S2CID 22529926.
- S2CID 45078589.
- S2CID 31966839.
- ^ S2CID 2834254.
- S2CID 34183578.
- PMID 15196509.
- PMID 21445631.
- ISBN 9780195340624.
- .
- )