Tiagabine
 | |
| Clinical data | |
|---|---|
| Pronunciation | /taɪˈæɡəbiːn/ |
| Trade names | Gabitril |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698014 |
| Pregnancy category |
|
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Onset of action | Tmax = 45 min[3] |
| Elimination half-life | 5–8 hours[4] |
| Excretion | Fecal (63%) and renal (25%)[3] |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Tiagabine (trade name Gabitril) is an
Medical uses
Tiagabine is approved by U.S.
The
Side effects
Side effects of tiagabine are dose related.
Warning
- CNS depression
- Dermatologic reactions
- Generalized weakness
- Ophthalmic effects
- Suicidal ideation[11]
Overdose
Tiagabine
Pharmacology
Tiagabine increases the level of
Pharmacodynamics
Tiagabine is primarily used as an anticonvulsant in the treatment of epilepsy as a supplement. Although the exact mechanism by which Tiagabine exerts its antiseizure effect is unknown, it is thought to be related to its ability to increase the activity of gamma aminobutyric acid (GABA), the central nervous system's major inhibitory neurotransmitter. Tiagabine attaches to the GABA uptake carrier's recognition sites. Tiagabine is thought to block GABA uptake into presynaptic neurons as a result of this action, allowing more GABA to be available for receptor binding on the surfaces of post-synaptic cells.[14]
Effects on cortical delta oscillations
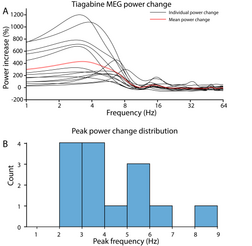
Tiagabine enhances the power of cortical delta (< 4 Hz) oscillations up to 1000% relative to placebo, which may result in an EEG or MEG signature resembling non-rapid eye movement sleep even while the person who has taken tiagabine is awake and conscious.[15] This demonstrates that cortical delta activity and wakeful consciousness are not mutually exclusive, i.e., high amplitude delta oscillations are not always a reliable indicator of unconsciousness.
Monitoring Parameters
Seizure frequency, liver function tests, suicidality[16]
History
Tiagabine was discovered at Novo Nordisk in Denmark in 1988 by a team of medicinal chemists and pharmacologists under the general direction of Claus Bræstrup.[17] The drug was co-developed with Abbott Laboratories, in a 40/60 cost sharing deal, with Abbott paying a premium for licensing the IP from the Danish company.[citation needed]
U.S. patents on tiagabine listed in the Orange Book expired in April 2016.[18]
See also
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ ISBN 978-1-60913-345-0.
- ^ a b c "Gabitril (tiagabine hydrochloride) Tablets. U.S. Full Prescribing Information" (PDF). Cephalon, Inc. Retrieved 8 April 2016.
- ^ S2CID 27336198.
- ^ ISBN 978-0-521-75900-7.
- ^ PMID 31643697, retrieved 2021-12-24
- PMID 27998379.
- ^ S2CID 24203401.
- ISBN 978-3-642-60072-2.
- ISBN 978-0-444-53266-4.
- S2CID 23614412.
- ^ S2CID 25469390.
- PMID 16420077.
- ^ "Gabitril (tiagabine) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 2021-12-24.
- PMID 37340024.
- S2CID 70426629.
- PMID 8510100.
- ^ "Search Results for Tiagabine". Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration. Archived from the original on 22 April 2016. Retrieved 22 March 2016.
External links
- Gabitril(manufacturer's website)
