Tin(II) fluoride
 Sn2+; F−
| |
| Names | |
|---|---|
| IUPAC name
Tin(II) fluoride
| |
| Other names
Stannous fluoride
| |
| Identifiers | |
3D model (
JSmol ) |
|
ECHA InfoCard
|
100.029.090 |
PubChem CID
|
|
RTECS number
|
|
| UNII | |
| UN number | 3288 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SnF2 | |
| Molar mass | 156.69 g/mol |
| Appearance | colorless solid |
| Density | 4.57 g/cm3 |
| Melting point | 213 °C (415 °F; 486 K) |
| Boiling point | 850 °C (1,560 °F; 1,120 K) |
| 31 g/100 mL (0 °C); 35 g/100 mL (20 °C); 78.5 g/100 mL (106 °C) | |
| Solubility | soluble in KOH, KF; negligible in ethanol, ether, chloroform |
| Structure | |
Monoclinic, mS48
| |
| C2/c, No. 15 | |
| Pharmacology | |
| A01AA04 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 0860 |
| Related compounds | |
Other anions
|
Tin(II) chloride, Tin(II) bromide, Tin(II) iodide |
Other cations
|
Lead(IV) fluoride
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin(II) fluoride, commonly referred to commercially as stannous fluoride[1][2] (from Latin stannum, 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
Oral health benefits
Stannous fluoride was introduced as an alternative to sodium fluoride for the prevention of cavities (tooth decay). It was introduced for this purpose by Joseph Muhler and William Nebergall. In recognition for their innovation, these two individuals were inducted into the Inventor's Hall of Fame.[1]
The fluoride in stannous fluoride helps to convert the calcium mineral apatite in teeth into fluorapatite, which makes tooth enamel more resistant to bacteria-generated acid attacks.[3] The calcium present in plaque and saliva reacts with fluoride to form calcium fluoride on the tooth surface; over time, this calcium fluoride dissolves to allow calcium and fluoride ions to interact with the tooth and form fluoride-containing apatite within the tooth structure.[4] This chemical reaction inhibits demineralisation and can promote remineralisation of tooth decay. The resulting fluoride-containing apatite is more insoluble, and more resistant to acid and tooth decay.[4]
In addition to fluoride, the stannous ion has benefits for oral health when incorporated in a toothpaste. At similar fluoride concentrations, toothpastes containing stannous fluoride have been shown to be more effective than toothpastes containing sodium fluoride for reducing the incidence of dental caries and
Stannous fluoride was once used under the
Production
SnF2 can be prepared by evaporating a solution of SnO in 40% HF.[20]
- SnO + 2 HF → SnF2 + H2O
Aqueous solutions
Readily soluble in water, SnF2 is hydrolysed. At low concentration, it forms species such as SnOH+, Sn(OH)2 and Sn(OH)3−. At higher concentrations, predominantly polynuclear species are formed, including Sn2(OH)22+ and Sn3(OH)42+.[21] Aqueous solutions readily oxidise to form insoluble precipitates of SnIV, which are ineffective as a dental prophylactic.[22] Studies of the oxidation using Mössbauer spectroscopy on frozen samples suggests that O2 is the oxidizing species.[23]
Lewis acidity
SnF2 acts as a
In solutions containing the fluoride ion, F−, it forms the fluoride complexes SnF3−, Sn2F5−, and SnF2(OH2).[26] Crystallization from an aqueous solution containing NaF produces compounds containing polynuclear anions, e.g. NaSn2F5 or Na4Sn3F10 depending on the reaction conditions, rather than NaSnF3.[20] The compound NaSnF3, containing the pyramidal SnF3− anion, can be produced from a pyridine–water solution.[27] Other compounds containing the pyramidal SnF3− anion are known, such as Ca(SnF3)2.[28]
Reducing properties
SnF2 is a reducing agent, with a standard reduction potential of Eo (SnIV/ SnII) = +0.15 V.[29] Solutions in HF are readily oxidised by a range of oxidizing agents (O2, SO2 or F2) to form the mixed-valence compound Sn3F8 (containing SnII and SnIV and no Sn–Sn bonds).[20]
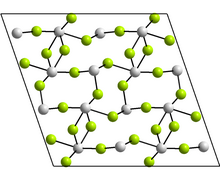
Structure
The monoclinic form contains tetramers, Sn4F8, where there are two distinct coordination environments for the Sn atoms. In each case, there are three nearest neighbours, with Sn at the apex of a trigonal pyramid, and the lone pair of electrons sterically active.[30] Other forms reported have the GeF2 and paratellurite structures.[30]
Molecular SnF2
In the vapour phase, SnF2 forms monomers, dimers, and trimers.[26] Monomeric SnF2 is a non-linear molecule with an Sn−F bond length of 206 pm.[26] Complexes of SnF2, sometimes called difluorostannylene, with an alkyne and aromatic compounds deposited in an argon matrix at 12 K have been reported.[31][32]
Safety
Stannous fluoride can cause redness and irritation if it is inhaled or comes into contact with the eyes. If ingested, it can cause abdominal pains and shock.[33] Rare but serious allergic reactions are possible; symptoms include itching, swelling, and difficulty breathing. Certain formulations of stannous fluoride in dental products may cause mild tooth discoloration; this is not permanent and can be removed by brushing, or can be prevented by using a stabilised stannous fluoride toothpaste.[15][16][34]
References
- ^ a b "National Inventors Hall of Fame Announces 2019 Inductees at CES" (Press release). National Inventors Hall of Fame. Retrieved 6 February 2019.
- ^ "Latin Names Variable Charge Metals". Nobel.SCAS.BCIT.ca/. British Columbia Institute of Technology Chemistry Department. Archived from the original on 22 July 2020. Retrieved 16 June 2013.
- PMID 1063601.
- ^ PMID 23192605.
- PMID 27477786.
- S2CID 45156274.
- PMID 30556372.
- PMID 32700515.
- S2CID 489634.
- PMID 32216778.
- S2CID 73488958.
- PMID 15642062.
- PMID 15732859.
- S2CID 220336087.
- ^ PMID 17410949.
- ^ PMID 31872105.
- PMID 8593190.
- PMID 8593191.
- S2CID 229940161.
- ^ ISBN 978-0-08-037941-8.
- .
- ISBN 978-0-7817-4673-1
- S2CID 96184099.
- .
- .
- ^ ISBN 0-12-352651-5.
- .
- S2CID 91430538.
- ISBN 978-0-13-039913-7.
- ^ ISBN 0-19-855370-6
- S2CID 97064510.
- S2CID 94004320.
- ^ "Stannous fluoride (International Chemical Safety Cards: 0860)". International Labour Organization. Retrieved June 21, 2021.
- ^ "Stannous Fluoride-Dental". WebMD. Retrieved March 11, 2014.

