trp operon

The trp operon is a group of genes that are transcribed together, encoding the enzymes that produce the amino acid tryptophan in bacteria. The trp operon was first characterized in Escherichia coli, and it has since been discovered in many other bacteria.[1] The operon is regulated so that, when tryptophan is present in the environment, the genes for tryptophan synthesis are repressed.
The trp operon contains five structural genes: trpE, trpD, trpC, trpB, and trpA, which encode the enzymes needed to synthesize tryptophan. It also contains a repressive
This operon is an example of
The trp operon is well-studied and is commonly used as an example of gene regulation in bacteria alongside the lac operon.
Genes
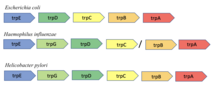
trp operon contains five structural genes. The roles of their products are:
- TrpE (P00895): anthranilate.
- TrpD (P00904): Cooperates with TrpE.
- TrpC (P00909): Phosphoribosylanthranilate isomerase domain first turns N-(5-phospho-β-D-ribosyl)anthranilate into 1-(2-carboxyphenylamino)-1-deoxy-D-ribulose 5-phosphate. The Indole-3-glycerol-phosphate synthase on the same protein then turns the product into (1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate.
- TrpA (P0A877), TrpB (P0A879): two subunits of tryptophan synthetase. Combines TrpC's product with serine to produce tryptophan.

Repression

The
Attenuation

At the beginning of the transcribed genes of the trp operon is a sequence of at least 130 nucleotides termed the leader transcript (trpL; P0AD92).
Part of the leader transcript codes for a short
This attenuation mechanism is experimentally supported. First, the translation of the leader peptide and ribosomal stalling are directly evidenced to be necessary for inhibiting the transcription termination.[13] Moreover, mutational analysis destabilizing or disrupting the base-pairing of the antiterminator hairpin results in increased termination of several folds; consistent with the attenuation model, this mutation fails to relieve attenuation even with starved Trp.[10][13] In contrast, complementary oligonucleotides targeting strand 1 increases the operon expression by promoting the antiterminator formation.[10][14] Furthermore, in histidine operon, compensatory mutation shows that the pairing ability of strands 2–3 matters more than their primary sequence in inhibiting attenuation.[10][15]
In attenuation, where the translating ribosome is stalled determines whether the termination hairpin will be formed.[10] In order for the transcribing polymerase to concomitantly capture the alternative structure, the time scale of the structural modulation must be comparable to that of the transcription.[2] To ensure that the ribosome binds and begins translation of the leader transcript immediately following its synthesis, a pause site exists in the trpL sequence. Upon reaching this site, RNA polymerase pauses transcription and apparently waits for translation to begin. This mechanism allows for synchronization of transcription and translation, a key element in attenuation.
A similar attenuation mechanism regulates the synthesis of histidine, phenylalanine and threonine.
Regulation of trp operon in Bacillus subtilis
The arrangement of the trp operon in E. coli and Bacillus subtilis differs. There are 5 structural genes in E. coli that are found under a single transcriptional unit. In Bacillus subtilis, there are 6 structural genes that are situated within a supraoperon. Three of these genes are found upstream while the other three genes are found downstream of the trp operon. [16] There is a 7th gene in Bacillus subtilis's operon called trpG or pabA which is responsible for protein synthesis of tryptophan and folate. [17] Regulation of trp operons in both organisms depends on the amount of trp present in the cell. However, the primary regulation of tryptophan biosynthesis in B. subtilis is via attenuation, rather than repression, of transcription.[18] In B. subtilis, tryptophan binds to the eleven-subunit tryptophan-activated RNA-binding attenuation protein (TRAP), which activates TRAP's ability to bind to the trp leader RNA.[19][20] Binding of trp-activated TRAP to leader RNA results in the formation of a terminator structure that causes transcription termination.[18] In addition, the activated TRAP inhibits the initiation of translation of trpP, trpE, trpG and ycbK genes. The gene trpP plays a role in trp transportation, while the gene trpG is utilized in the folate operon, and the gene ycbK is involved in synthesis of an efflux protein. The activated TRAP protein is regulated by an anti-TRAP protein and AT synthesis. AT can inactive TRAP to lower the transcription of tryptophan.[21]
References
- PMID 18374625.
- ^ S2CID 4364204.
- ISBN 978-0-7167-7108-1.
- PMID 781269.
- ^ PMID 337297.
- PMID 118451.
- S2CID 4347442.
- PMID 6159477.
- PMID 368800.
- ^ PMID 6186194.
- PMID 351195.
- PMID 364484.
- ^ PMID 366606.
- ^ PMID 6179092.
- ^ PMID 6167727.
- PMID 15262409.
- PMID 15743934.
- ^ PMID 16285852.
- PMID 11214184.
- S2CID 4340136.
- PMID 18178730.
Further reading
- Morse DE, Mosteller RD, Yanofsky C (1969). "Dynamics of synthesis, translation, and degradation of trp operon messenger RNA in E. coli". Cold Spring Harbor Symposia on Quantitative Biology. 34: 725–40. PMID 4909527.
- Yanofsky C (February 1981). "Attenuation in the control of expression of bacterial operons". Nature. 289 (5800): 751–8. S2CID 4364204.
