Uterine fibroid
| Uterine fibroids | |
|---|---|
| Other names | Uterine leiomyoma, uterine myoma, myoma, fibromyoma, fibroleiomyoma |
gonadotropin releasing hormone agonist[1] | |
| Prognosis | Improve after menopause[1] |
| Frequency | ~50% of women by age 50[1] |
Uterine fibroids, also known as uterine leiomyomas or fibroids, are
The exact cause of uterine fibroids is unclear.[1] However, fibroids run in families and appear to be partly determined by hormone levels.[1] Risk factors include obesity and eating red meat.[1] Diagnosis can be performed by pelvic examination or medical imaging.[1]
Treatment is typically not needed if there are no symptoms.
About 20% to 80% of women develop fibroids by the age of 50.[1] In 2013, it was estimated that 171 million women were affected worldwide.[5] They are typically found during the middle and later reproductive years.[1] After menopause, they usually decrease in size.[1] In the United States, uterine fibroids are a common reason for surgical removal of the uterus.[6]
Signs and symptoms
Some women with uterine fibroids do not have symptoms. Abdominal pain, anemia and increased bleeding can indicate the presence of fibroids.
While fibroids are common, they are not a typical cause for infertility, accounting for about 3% of reasons why a woman may not be able to have a child.
Risk factors
Some risk factors associated with the development of uterine fibroids are modifiable.[12] Fibroids are more common in obese women.[13] Fibroids are dependent on estrogen and progesterone to grow and therefore relevant only during the reproductive years.
Diet
Diets high in fruits and vegetables tend to lower the risk of developing fibroids.[12] Fibers, vitamin A, C and E, phytoestrogens, carotenoids, meat, fish, and dairy products are of unclear effect.[12] Normal dietary levels of vitamin D may reduce the risk of developing fibroids.[12]
Genetics
Fifty percent of uterine fibroids demonstrate a genetic abnormality. Often a translocation is found on some chromosomes.[7] Fibroids are partly genetic. If a mother had fibroids, risk in the daughter is about three times higher than average.[14] Black women have a 3–9 times increased chance of developing uterine fibroids than white women.[15] Only a few specific genes or cytogenetic deviations are associated with fibroids. 80–85% of fibroids have a mutation in the mediator complex subunit 12 (MED12) gene.[16][17]
Familial leiomyomata
A syndrome (
Pathophysiology

Fibroids are a type of uterine leiomyoma. Fibroids grossly appear as round, well circumscribed (but not encapsulated), solid nodules that are white or tan, and show whorled appearance on histological section. The size varies, from microscopic to lesions of considerable size. Typically lesions the size of a grapefruit or bigger are felt by the patient herself through the abdominal wall.[1]

Microscopically, tumor cells resemble normal cells (elongated, spindle-shaped, with a cigar-shaped nucleus) and form bundles with different directions (whorled). These cells are uniform in size and shape, with scarce mitoses. There are three benign variants: bizarre (atypical); cellular; and mitotically active.
The appearance of prominent nucleoli with peri-nucleolar halos should alert the pathologist to investigate the possibility of the extremely rare hereditary leiomyomatosis and renal cell cancer (Reed) syndrome.[21]
Location and classification

Growth and location are the main factors that determine if a fibroid leads to symptoms and problems.[6] A small lesion can be symptomatic if located within the uterine cavity while a large lesion on the outside of the uterus may go unnoticed. Different locations are classified as follows:
- Intramural fibroids are located within the muscular wall of the uterus and are the most common type.[22] Unless they are large, they may be asymptomatic. Intramural fibroids begin as small nodules in the muscular wall of the uterus. With time, intramural fibroids may expand inwards, causing distortion and elongation of the uterine cavity.
- Subserosal fibroids are located on the surface of the uterus. They can also grow outward from the surface and remain attached by a small piece of tissue and then are called pedunculated fibroids.[1]
- Submucosal fibroids are located in the muscle beneath the endometrium of the uterus and distort the uterine cavity; even small lesions in this location may lead to bleeding and infertility. A pedunculated lesion within the cavity is termed an intracavitary fibroid and can be passed through the cervix.
- Cervical fibroids are located in the wall of the cervix (neck of the uterus). Rarely, fibroids are found in the supporting structures (broad ligament, or uterosacral ligament) of the uterus that also contain smooth muscle tissue.
Since 2011 the FIGO published their consensus paper on the classification of fibroids, namely from 0 to 8. This is part of the PALM COEIN classification and is the most frequently used in clinical practise and research [23] Please continue reading here: FIGO classification.
- Type 0: pedunculated submucosal, intracavitary (i.e. inside of the uterus)
- Type 1: submucosal, <50% intramural
- Type 2: submucosal, ≥50% intramural
- Type 3: contacts the endometrium, 100% intramural
- Type 4: intramural (i.e. completely inside the wall of the uterus)
- Type 5: subserosal, ≥50% intramural
- Type 6: subserosal, <50% intramural
- Type 7: pedunculated subserosal
- Type 8: other (e.g. cervcial, parasitic)
There are also hybrid leimyomas, like the type 2-5 which are both subserosal as submucosal.
Fibroids may be single or multiple. Most fibroids start in the muscular wall of the uterus. With further growth, some lesions may develop towards the outside of the uterus or towards the internal cavity. Secondary changes that may develop within fibroids are hemorrhage, necrosis, calcification, and cystic changes. They tend to calcify after menopause.[24]
If the uterus contains too many to count, it is referred to as diffuse uterine leiomyomatosis.
Extrauterine fibroids of uterine origin, metastatic fibroids
Fibroids of uterine origin located in other parts of the body, sometimes also called parasitic myomas have been historically extremely rare, but are now diagnosed with increasing frequency. They may be related or identical to metastasizing leiomyoma.
They are in most cases still hormone dependent but may cause life-threatening complications when they appear in distant organs. Some sources suggest that a substantial share of the cases may be late complications of surgeries such as myomectomy or hysterectomy. Particularly laparoscopic myomectomy using a morcellator has been associated with an increased risk of this complication.[25][26][27]
There are a number of rare conditions in which fibroids metastasize. They still grow in a benign fashion, but can be dangerous depending on their location.[28]
- In leiomyoma with vascular invasion, an ordinary-appearing fibroid invades into a vessel but there is no risk of recurrence.
- In intravenous leiomyomatosis, leiomyomata grow in veins with uterine fibroids as their source. Involvement of the heart can be fatal.
- In benign metastasizing leiomyoma, leiomyomata grow in more distant sites such as the lungs and lymph nodes. The source is not entirely clear. Pulmonary involvement can be fatal.
- In disseminated intraperitoneal leiomyomatosis, leiomyomata grow diffusely on the peritoneal and omental surfaces, with uterine fibroids as their source. This can simulate a malignant tumor but behaves benignly.
Pathogenesis



Fibroids are
The exact cause of fibroids is not clearly understood, but the current working hypothesis is that genetic predispositions, prenatal hormone exposure and the effects of hormones, growth factors and
It is believed that estrogen and progesterone have a
Aromatase and 17beta-hydroxysteroid dehydrogenase are aberrantly expressed in fibroids, indicating that fibroids can convert circulating androstenedione into estradiol.[33] Similar mechanism of action has been elucidated in endometriosis and other endometrial diseases.[34] Aromatase inhibitors are currently considered for treatment, at certain doses they would completely inhibit estrogen production in the fibroid while not largely affecting ovarian production of estrogen (and thus systemic levels of it). Aromatase overexpression is particularly pronounced in African-American women.[35]
Genetic and hereditary causes are being considered and several epidemiologic findings indicate considerable genetic influence especially for early onset cases. First degree relatives have a 2.5-fold risk, and nearly 6-fold risk when considering early onset cases.
Expansion of uterine fibroids occurs by a slow rate of cell proliferation combined with the production of copious amounts of extracellular matrix.[35]
A small population of the cells in a uterine fibroid have properties of stem cells or progenitor cells, and contribute significantly to ovarian steroid-dependent growth of fibroids. These stem-progenitor cells are deficient in estrogen receptor α and progesterone receptor and instead rely on substantially higher levels of these receptors in surrounding differentiated cells to mediate estrogen and progesterone actions via paracrine signaling.[35]
Diagnosis
Physical examination and ultrasound are sufficient for diagnosing uterine fibroids in the majority of patients. When ultrasound findings are inconclusive, magnetic resonance imaging (MRI) may be able to confirm the diagnosis of uterine fibroids in most cases. In addition, MRI can identify benign uterine fibroids with atypical imaging features and fibroids with variant growth patterns. MRI can also identify other uterine (e.g. adenomyosis, endometrial polyps, endometrial cancer) and extrauterine (e.g. benign and malignant ovarian tumors, endometriosis) disorders that may mimic the appearance of uterine fibroids and/or contribute to the patient's symptoms.[37] However, a small proportion of uterine fibroids can mimic other malignant uterine tumors (e.g. leiomyosarcoma) on all available imaging modalities (e.g. ultrasound, CT, MRI and PET-CT).[37]
Malignant tumors of the uterine wall (e.g. leiomyosarcoma) are very rare. Findings suggestive of a malignant uterine tumor rather than a benign fibroid include, fast or unexpected growth (particularly after menopause), interruption/effacement of the endometrial stripe, lymph node enlargement, invasion of adjacent organs and metastases to distant organs (e.g. lung). MRI findings suggestive of a malignancy include nodular/ill-circumscribed tumor margins, intermediate/high T2-weighted signal intensity of the solid tumor components, regions with high signal T1-weighted sequences in keeping with subacute hemorrhage, fine/wispy enhancement of the solid parts of the tumor, and restricted diffusion on diffusion-weighted imaging (DWI).[37] A biopsy is rarely performed and if performed, is rarely diagnostic. Should there be an uncertain diagnosis after ultrasounds and MRI imaging, surgery is generally indicated.[38]
-
A very large (9 cm) fibroid of the uterus which is causing pelvic congestion syndrome as seen on CT
-
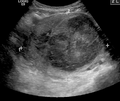
A very large (9 cm) fibroid of the uterus which is causing pelvic congestion syndrome as seen on ultrasound
-
A relatively large submucosal leiomyoma; it fills out the major part of the endometrial cavity.
-
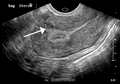
A small uterine fibroid seen within the wall of the myometrium on a cross-sectional ultrasound view
-
Two calcified fibroids (in the uterus)
-
A subserosal uterine fibroid with a diameter of 5 centimeters
-
MRI image with multiple uterine leiomyomas
-
Giant leiomyomas almost filling the abdomen
-
Histopathology of uterine fibroids typically show smooth muscle in a whorled (fascicular) pattern.[39]
-
This variant of Van Gieson's stain distinguishes muscle (yellow) from connective tissue (red).
-
β-cateninin uterine leiomyoma, which is negative as there is only staining of cytoplasm but not of cell nuclei.
Negative
Coexisting disorders
Fibroids that lead to heavy vaginal bleeding lead to
In very rare cases, malignant (cancerous) growths,
Treatment
Most fibroids do not require treatment unless they are causing symptoms. After menopause, fibroids shrink, and it is unusual for them to cause problems.
Symptomatic uterine fibroids can be treated by:
- medication to control symptoms (i.e., symptomatic management)
- medication aimed at shrinking tumors
- ultrasound fibroid destruction
- myomectomy or radiofrequency ablation
- hysterectomy
- uterine artery embolization
In those who have symptoms, uterine artery embolization and surgical options have similar outcomes with respect to satisfaction.[41]
For decades, a common approach to treating symptomatic fibroids was "either get a hysterectomy or wait until menopause diminishes the symptoms," but minimally invasive and noninvasive options were often not offered.[42] Especially since the 2010s, minimally invasive and noninvasive options are increasingly being offered as they have advanced on their technological journey from being new and unusual to being common clinical practice.[42]
Medication
A number of medications may be used to control symptoms.
Cabergoline in a moderate and well-tolerated dose has been shown in two studies to shrink fibroids effectively. The mechanism of action responsible for how cabergoline shrinks fibroids is unclear.[44]
Ulipristal acetate is a synthetic selective progesterone receptor modulator (SPRM) that has tentative evidence to support its use for presurgical treatment of fibroids with low side-effects.[46] Long-term UPA-treated fibroids have shown volume reduction of about 70%.[47] In some cases UPA alone is used to relieve symptoms without surgery,[48] and to allow successful pregnancies without fibroid regrowth.[49] Indeed, in the tumor cells, the molecule blocks the cell proliferation, induces their apoptosis[50][51] and stimulates the remodeling of the extensive fibrosis by matrix metalloproteinases,[52] hence explaining the long-term benefit.[53] Yet, due to some rare but severe hepatic injuries after UPA treatment, the licence was suspended in 2020 in the EU[54] and voluntary removed in Canada.[55]
Danazol is an effective treatment to shrink fibroids and control symptoms. Its use is limited by unpleasant side effects. Mechanism of action is thought to be antiestrogenic effects. Recent experience indicates that safety and side effect profile can be improved by more cautious dosing.[44]
Progesterone antagonists such as mifepristone have been tested, there is evidence that it relieves some symptoms and improves quality of life but because of adverse histological changes that have been observed in several trials it can not be currently recommended outside of research setting.[56] Fibroid growth has recurred after antiprogestin treatment was stopped.[35]
Aromatase inhibitors have been used experimentally to reduce fibroids. The effect is believed to be due partially by lowering systemic estrogen levels and partially by inhibiting locally overexpressed aromatase in fibroids.[44] However, fibroid growth has recurred after treatment was stopped.[35] Experience from experimental aromatase inhibitor treatment of endometriosis indicates that aromatase inhibitors might be particularly useful in combination with a progestogenic ovulation inhibitor.
Uterine artery
Uterine artery embolization (UAE) is a noninvasive procedure that blocks blood flow to fibroids, causing them to shrink.[57] Long-term outcomes with respect to how happy people are with the procedure are similar to that of surgery.[58] There is tentative evidence that traditional surgery may result in better fertility.[58] One review found that UAE doubles the future risk of miscarriage.[59] UAE also appears to require more repeat procedures than if surgery was done initially.[58] A person will usually recover from the procedure within a few days.
Uterine artery ligation, sometimes also laparoscopic occlusion of uterine arteries are minimally invasive methods to limit blood supply of the uterus by a small surgery that can be performed transvaginally or laparoscopically. The principal mechanism of action may be similar like in UAE but is easier to perform and fewer side effects are expected.[60][non-primary source needed][61][non-primary source needed]
The 2016 NICE (National Institute of Clinical Excellence – the non governmental public body that publishes guidelines in the use of health technologies and good clinical practice in the United Kingdom) guidelines state UAE/UFE can be offered to women with symptomatic fibroids (fibroids being usually >30mm in size). Women should be informed that UAE and myomectomy (the surgical removal of fibroids) may potentially allow them to retain their fertility.[62]
Myomectomy

Myomectomy is a surgery to remove one or more fibroids. It is usually recommended when more conservative treatment options fail for women who want fertility preserving surgery or who want to retain the uterus.[63]
There are three types of myomectomy:
- In a hysteroscopic myomectomy (also called transcervical resection), the fibroid can be removed by either the use of a resectoscope, an endoscopic instrument inserted through the vagina and cervix that can use high-frequency electrical energy to cut tissue, or a similar device.
- A morbidity rates and faster recovery than does laparotomic myomectomy.[64]
- A laparotomic myomectomy (also known as an open or abdominal myomectomy) is the most invasive surgical procedure to remove fibroids. The physician makes an incision in the abdominal wall and removes the fibroids from the uterus.
Laparoscopic myomectomy has less pain and shorter time in hospital than open surgery.[65] An analysis of 15,000 women found that those who had myomectomy required fewer additional procedures to mange fibroids (including hysterectomies) over the next 5 years than those who had UAE.[66][67]
Hysterectomy
Hysterectomy was the classical method of treating fibroids. Although it is now recommended only as last option, fibroids are still the leading cause of hysterectomies in the US.
Endometrial ablation
Endometrial ablation can be used if the fibroids are only within the uterus and not intramural and relatively small. High failure and recurrence rates are expected in the presence of larger or intramural fibroids.
Other procedures
Radiofrequency ablation is a minimally invasive treatments for fibroids.[68] In this technique the fibroid is shrunk by inserting a needle-like device into the fibroid through the abdomen and heating it with radio-frequency (RF) electrical energy to cause necrosis of cells. The treatment is a potential option for women who have fibroids, have completed child-bearing and want to avoid a hysterectomy.
Prognosis
About 1 out of 1,000 lesions are or become malignant, typically as a leiomyosarcoma on histology.[10] A sign that a lesion may be malignant is growth after menopause.[10] There is no consensus among pathologists regarding the transformation of leiomyoma into a sarcoma.
Metastasis
There are a number of rare conditions in which fibroids metastasize. They still grow in a benign fashion, but can be dangerous depending on their location.[28]
Epidemiology
About 20% to 80% of women develop fibroids by the age of 50.[12][1] Globally in 2013 it was estimated that 171 million women were affected.[5] They are typically found during the middle and later reproductive years.[1] After menopause they usually decrease in size.[1] Surgery to remove uterine fibroids occurs more frequently in women in "higher social classes".[12] Adolescents develop uterine fibroids much less frequently than older women.[7] Up to 50% of women with uterine fibroids have no symptoms. The prevalence of uterine fibroids among teenagers is 0.4%.[7]
Europe
The incidence of uterine fibroids in Europe is thought to be lower than the incidence in the US.[12]
United States
Eighty percent of African American women will develop benign uterine fibroid tumors by their late 40s, according to the National Institute of Environmental Health Sciences.[73] African American women are two to three times more likely to get fibroids than Caucasian women.[12][13][74] In African American women fibroids seem to occur at a younger age, grow more quickly, and are more likely to cause symptoms.[75] This leads to higher rates of surgery for African Americans, both myomectomy, and hysterectomy.[76] Increased risk of fibroids in African Americans causes them to fare worse in in-vitro fertility treatments and raises their risk of premature births and delivery by Caesarean section.[76]
It is unclear why fibroids are more common in African American women. Some studies suggest that black women who are obese and who have
Related legislation
United States
The 2005 S.1289 bill was read twice and referred to the committee on Health, Labor, and Pensions but never passed for a Senate or House vote; the proposed Uterine Fibroid Research and Education Act of 2005 mentioned that $5 billion is spent annually on hysterectomy surgeries each year, which affect 22% of African Americans and 7% of Caucasian women. The bill also called for more funding for research and educational purposes. It also states that of the $28 billion issued to NIH, $5 million was allocated for uterine fibroids in 2004.[77]
Other animals
Uterine fibroids are rare in other mammals, although they have been observed in certain dogs and Baltic grey seals.[78]
Research
Notes
- ^ Women make up the majority of people who can develop uterine fibroids, with trans men and non-binary people who were observed female at birth making up the remainder; a person must have a uterus to develop these fibroids.
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak "Uterine fibroids fact sheet". Office on Women's Health. January 15, 2015. Archived from the original on 7 July 2015. Retrieved 26 June 2015.
- ISBN 978-0-323-07699-9.
- ^ "Uterine Fibroids | Fibroids | MedlinePlus". Retrieved 2018-11-07.
- PMID 26796059.
- ^ PMID 26063472.
- ^ S2CID 45013410.
- ^ PMID 25609056.
- ISBN 978-1-4471-4953-8.
- ^ "Uterine Fibroids: Symptoms, Causes, Risk Factors & Treatment". Cleveland Clinic. Retrieved 2022-10-08.
- ^ a b c d e American Society of Reproductive Medicine Patient Booklet: Uterine Fibroids, 2003 Archived 2008-07-03 at the Wayback Machine
- PMID 24401287.
- ^ S2CID 35376265.
- ^ a b Uterine Fibroids at Merck Manual of Diagnosis and Therapy Professional Edition
- ^ "Uterine fibroids fact sheet". womenshealth.gov. 2016-12-15. Archived from the original on 2016-02-09.
- .
- S2CID 13931280.
- PMID 28432313.
- PMID 22473397.
- PMID 12772087.
- ^ "Reed syndrome". Archived from the original on 2012-02-24. Retrieved 2012-04-09.[full citation needed]
- S2CID 1342593.
- ^ a b "Fibroids". NHS Choices. U.K. National Health Service. 2017-10-19. Archived from the original on 2008-05-05.
- S2CID 205260568.
- ISBN 978-1-119-01080-7.
- PMID 21719004.
- PMID 20580324.
- ^ "FDA Updated Assessment of The Use of Laparoscopic Power Morcellators to Treat Uterine Fibroids" (PDF). Food and Drug Administration. Retrieved 23 December 2017.
- ^ a b Fletcher's Diagnostic Histopathology of Tumors (3rd ed.). pp. 692–4.
- S2CID 20288929.
- PMID 18534913.
- PMID 15140868.
- ^ PMID 19524896.
- S2CID 260319833.
- S2CID 7642211.
- ^ PMID 25205766.
- PMID 17613550.
- ^ S2CID 248725335.
- ^ "Uterine fibroids - Diagnosis and treatment - Mayo Clinic". www.mayoclinic.org. Retrieved 2022-10-08.
- ^ Mohamed Mokhtar Desouki. "Uterus - Stromal tumors - Leiomyoma". pathology Outlines. Topic Completed: 1 August 2011. Revised: 15 December 2019
- PMID 33999334.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - PMID 25541260.
- ^ a b Cimons M (2022-12-11). "Fibroids are serious. Surgery isn't the only way to stop them. Many women are not told about noninvasive treatments that don't affect fertility. Experts want to change that". Washington Post. Retrieved 2022-12-11.
- PMID 20682142.
- ^ PMID 18468953.
- PMID 19920976.
- S2CID 25905881.
- S2CID 25077228.
- PMID 22296076.
- PMID 25241376.
- PMID 26003270.
- S2CID 44106868.
- PMID 29408988.
- PMID 26477496.
- ^ "European Medicament Agency".
- ^ Government of Canada, Health Canada (2020-09-16). "Allergan Inc. voluntarily withdraws its drug Fibristal, used to treat uterine fibroids, from the Canadian market". healthycanadians.gc.ca. Retrieved 2021-03-17.
- PMID 22895965.
- ^ "The Embolisation Process". FEmISA: Fibroid Embolisation: Information, Support, Advice. Archived from the original on 2014-05-31.
- ^ PMID 25541260.
- PMID 19361799.
- PMID 11172850.
- S2CID 8042317.
- ^ "Uterine Fibroid Embolisation Africa". 2017-02-23.
- PMID 31995657.
- PMID 18325839.
- PMID 25331441.
- S2CID 256325720.
- S2CID 262209805.
- ^ Beck M (2010-01-20). "A New Treatment to Help Women Avoid Hysterectomy". The Wall Street Journal.
- ^ "FDA Approves New Device to Treat Uterine Fibroids" (Press release). FDA. 2004-10-22. Archived from the original on 2008-05-09. Retrieved 2008-05-26.
- PMID 19358440.
- PMID 16412721.
- PMID 25932673.
- ^ "Helping Black Women Recognize, Treat Fibroids". NPR. Archived from the original on 22 January 2012. Retrieved 30 March 2011.
- ^ "African American Women and Fibroids". Philadelphia Black Women's Health Project. Archived from the original on 1 April 2011. Retrieved 30 March 2011.
- ^ "Minority Women's Health". Women's Health.gov. Archived from the original on 2010-08-30.
- ^ a b c "Black Women and High Prevalence of Fibroids". Fibroid Treatment Collective. November 29, 2010. Archived from the original on 25 December 2010. Retrieved 30 March 2011.
- ^ Office of Budget (PDF)Archived 2011-09-30 at the Wayback Machine[full citation needed]
- PMID 12637757.







![Histopathology of uterine fibroids typically show smooth muscle in a whorled (fascicular) pattern.[39]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/79/Histopathology_of_uterine_leiomyoma.jpg/120px-Histopathology_of_uterine_leiomyoma.jpg)

