Vacuum

A vacuum (pl.: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective vacuus (neuter vacuum) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure.[1] Physicists often discuss ideal test results that would occur in a perfect vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is considerably lower than atmospheric pressure.[2] The Latin term in vacuo is used to describe an object that is surrounded by a vacuum.
The quality of a partial vacuum refers to how closely it approaches a perfect vacuum. Other things equal, lower gas pressure means higher-quality vacuum. For example, a typical vacuum cleaner produces enough suction to reduce air pressure by around 20%.[3] But higher-quality vacuums are possible. Ultra-high vacuum chambers, common in chemistry, physics, and engineering, operate below one trillionth (10−12) of atmospheric pressure (100 nPa), and can reach around 100 particles/cm3.[4] Outer space is an even higher-quality vacuum, with the equivalent of just a few hydrogen atoms per cubic meter on average in intergalactic space.[5]
Vacuum has been a frequent topic of
Vacuum became a valuable industrial tool in the 20th century with the introduction of incandescent light bulbs and vacuum tubes, and a wide array of vacuum technologies has since become available. The development of human spaceflight has raised interest in the impact of vacuum on human health, and on life forms in general.
Etymology
The word vacuum comes from
Vacuum is one of the few words in the English language that contains two consecutive instances of the vowel u.[8]
Historical understanding
Historically, there has been much dispute over whether such a thing as a vacuum can exist. Ancient
In his Physics, book IV, Aristotle offered numerous arguments against the void: for example, that motion through a medium which offered no impediment could continue ad infinitum, there being no reason that something would come to rest anywhere in particular. Lucretius argued for the existence of vacuum in the first century BC and Hero of Alexandria tried unsuccessfully to create an artificial vacuum in the first century AD.[9]
In the medieval
European scholars such as Roger Bacon, Blasius of Parma and Walter Burley in the 13th and 14th century focused considerable attention on issues concerning the concept of a vacuum. Eventually following Stoic physics in this instance, scholars from the 14th century onward increasingly departed from the Aristotelian perspective in favor of a supernatural void beyond the confines of the cosmos itself, a conclusion widely acknowledged by the 17th century, which helped to segregate natural and theological concerns.[15]
Almost two thousand years after Plato, René Descartes also proposed a geometrically based alternative theory of atomism, without the problematic nothing–everything dichotomy of void and atom. Although Descartes agreed with the contemporary position, that a vacuum does not occur in nature, the success of his namesake coordinate system and more implicitly, the spatial–corporeal component of his metaphysics would come to define the philosophically modern notion of empty space as a quantified extension of volume. By the ancient definition however, directional information and magnitude were conceptually distinct.

Medieval

The 17th century saw the first attempts to quantify measurements of partial vacuum.[18] Evangelista Torricelli's mercury barometer of 1643 and Blaise Pascal's experiments both demonstrated a partial vacuum.
In 1654,
While outer space provides the most rarefied example of a naturally occurring partial vacuum, the heavens were originally thought to be seamlessly filled by a rigid indestructible material called aether. Borrowing somewhat from the pneuma of Stoic physics, aether came to be regarded as the rarefied air from which it took its name, (see Aether (mythology)). Early theories of light posited a ubiquitous terrestrial and celestial medium through which light propagated. Additionally, the concept informed Isaac Newton's explanations of both refraction and of radiant heat.[20] 19th century experiments into this luminiferous aether attempted to detect a minute drag on the Earth's orbit. While the Earth does, in fact, move through a relatively dense medium in comparison to that of interstellar space, the drag is so minuscule that it could not be detected. In 1912, astronomer Henry Pickering commented: "While the interstellar absorbing medium may be simply the ether, [it] is characteristic of a gas, and free gaseous molecules are certainly there".[21]
Later, in 1930, Paul Dirac proposed a model of the vacuum as an infinite sea of particles possessing negative energy, called the Dirac sea. This theory helped refine the predictions of his earlier formulated Dirac equation, and successfully predicted the existence of the positron, confirmed two years later. Werner Heisenberg's uncertainty principle, formulated in 1927, predicted a fundamental limit within which instantaneous position and momentum, or energy and time can be measured. This has far reaching consequences on the "emptiness" of space between particles. In the late 20th century, so-called virtual particles that arise spontaneously from empty space were confirmed.[citation needed]
Classical field theories
This subsection needs additional citations for verification. (April 2014) |
The strictest criterion to define a vacuum is a region of space and time where all the components of the stress–energy tensor are zero. This means that this region is devoid of energy and momentum, and by consequence, it must be empty of particles and other physical fields (such as electromagnetism) that contain energy and momentum.
Gravity
This subsection needs additional citations for verification. (April 2014) |
In
Electromagnetism
In
In the theory of classical electromagnetism, free space has the following properties:
- Electromagnetic radiation travels, when unobstructed, at the SI units.[27]
- The superposition principle is always exactly true.[28] For example, the electric potential generated by two charges is the simple addition of the potentials generated by each charge in isolation. The value of the electric field at any point around these two charges is found by calculating the vector sum of the two electric fields from each of the charges acting alone.
- The SI units), or exactly 1 (in Gaussian units).
- The characteristic impedance (η) equals the impedance of free space Z0 ≈ 376.73 Ω.[31]
The vacuum of classical electromagnetism can be viewed as an idealized electromagnetic medium with the constitutive relations in SI units:[32]
relating the
Quantum mechanics
In quantum mechanics and quantum field theory, the vacuum is defined as the state (that is, the solution to the equations of the theory) with the lowest possible energy (the ground state of the Hilbert space). In quantum electrodynamics this vacuum is referred to as 'QED vacuum' to distinguish it from the vacuum of quantum chromodynamics, denoted as QCD vacuum. QED vacuum is a state with no matter particles (hence the name), and no photons. As described above, this state is impossible to achieve experimentally. (Even if every matter particle could somehow be removed from a volume, it would be impossible to eliminate all the blackbody photons.) Nonetheless, it provides a good model for realizable vacuum, and agrees with a number of experimental observations as described next.
QED vacuum has interesting and complex properties. In QED vacuum, the electric and magnetic fields have zero average values, but their variances are not zero.
Theoretically, in QCD multiple vacuum states can coexist.[35] The starting and ending of cosmological inflation is thought to have arisen from transitions between different vacuum states. For theories obtained by quantization of a classical theory, each stationary point of the energy in the configuration space gives rise to a single vacuum. String theory is believed to have a huge number of vacua – the so-called string theory landscape.
Outer space

Outer space has very low density and pressure, and is the closest physical approximation of a perfect vacuum. But no vacuum is truly perfect, not even in interstellar space, where there are still a few hydrogen atoms per cubic meter.[5]
Stars, planets, and moons keep their atmospheres by gravitational attraction, and as such, atmospheres have no clearly delineated boundary: the density of atmospheric gas simply decreases with distance from the object. The Earth's atmospheric pressure drops to about 32 millipascals (4.6×10−6 psi) at 100 kilometres (62 mi) of altitude,[36] the Kármán line, which is a common definition of the boundary with outer space. Beyond this line, isotropic gas pressure rapidly becomes insignificant when compared to radiation pressure from the Sun and the dynamic pressure of the solar winds, so the definition of pressure becomes difficult to interpret. The thermosphere in this range has large gradients of pressure, temperature and composition, and varies greatly due to space weather. Astrophysicists prefer to use number density to describe these environments, in units of particles per cubic centimetre.
But although it meets the definition of outer space, the atmospheric density within the first few hundred kilometers above the Kármán line is still sufficient to produce significant
All of the observable universe is filled with large numbers of photons, the so-called cosmic background radiation, and quite likely a correspondingly large number of neutrinos. The current temperature of this radiation is about 3 K (−270.15 °C; −454.27 °F).
Measurement
The quality of a vacuum is indicated by the amount of matter remaining in the system, so that a high quality vacuum is one with very little matter left in it. Vacuum is primarily measured by its
Vacuum quality is subdivided into ranges according to the technology required to achieve it or measure it. These ranges were defined in ISO 3529-1:2019 as shown in the following table (100 Pa corresponds to 0.75 Torr; Torr is a non-SI unit):
| Pressure range | Definition | The reasoning for the definition of the ranges is as follows (typical circumstances): |
|---|---|---|
| Prevailing atmospheric pressure (31 kPa to 110 kPa) to 100 Pa | low (rough) vacuum | Pressure can be achieved by simple materials (e.g. regular steel) and positive displacement vacuum pumps; viscous flow regime for gases |
| <100 Pa to 0.1 Pa | medium (fine) vacuum | Pressure can be achieved by elaborate materials (e.g. stainless steel) and positive displacement vacuum pumps; transitional flow regime for gases |
| <0.1 Pa to 1×10−6 Pa | high vacuum (HV) | Pressure can be achieved by elaborate materials (e.g. stainless steel), elastomer sealings and high vacuum pumps; molecular flow regime for gases |
| <1×10−6 Pa to 1×10−9 Pa | ultra-high vacuum (UHV) | Pressure can be achieved by elaborate materials (e.g. low-carbon stainless steel), metal sealings, special surface preparations and cleaning, bake-out and high vacuum pumps; molecular flow regime for gases |
| below 1×10−9 Pa | extreme-high vacuum (XHV) | Pressure can be achieved by sophisticated materials (e.g. vacuum fired low-carbon stainless steel, aluminium, copper-beryllium, titanium), metal sealings, special surface preparations and cleaning, bake-out and additional getter pumps; molecular flow regime for gases |
- Atmospheric pressure is variable but 101.325 kilopascals (760 Torr) and 100 kilopascals (1000 mbar) are common standard or reference pressures.
- Deep space is generally much more empty than any artificial vacuum. It may or may not meet the definition of high vacuum above, depending on what region of space and astronomical bodies are being considered. For example, the MFP of interplanetary space is smaller than the size of the Solar System, but larger than small planets and moons. As a result, solar winds exhibit continuum flow on the scale of the Solar System, but must be considered a bombardment of particles with respect to the Earth and Moon.
- Perfect vacuum is an ideal state of no particles at all. It cannot be achieved in a quantum vacuum.
Relative versus absolute measurement
Vacuum is measured in units of pressure, typically as a subtraction relative to ambient atmospheric pressure on Earth. But the amount of relative measurable vacuum varies with local conditions. On the surface of Venus, where ground-level atmospheric pressure is much higher than on Earth, much higher relative vacuum readings would be possible. On the surface of the Moon with almost no atmosphere, it would be extremely difficult to create a measurable vacuum relative to the local environment.
Similarly, much higher than normal relative vacuum readings are possible deep in the Earth's ocean. A submarine maintaining an internal pressure of 1 atmosphere submerged to a depth of 10 atmospheres (98 metres; a 9.8-metre column of seawater has the equivalent weight of 1 atm) is effectively a vacuum chamber keeping out the crushing exterior water pressures, though the 1 atm inside the submarine would not normally be considered a vacuum.
Therefore, to properly understand the following discussions of vacuum measurement, it is important that the reader assumes the relative measurements are being done on Earth at sea level, at exactly 1 atmosphere of ambient atmospheric pressure.
Measurements relative to 1 atm

The
In other words, most low vacuum gauges that read, for example 50.79 Torr. Many inexpensive low vacuum gauges have a margin of error and may report a vacuum of 0 Torr but in practice this generally requires a two-stage rotary vane or other medium type of vacuum pump to go much beyond (lower than) 1 torr.
Measuring instruments
Many devices are used to measure the pressure in a vacuum, depending on what range of vacuum is needed.[39]
Hydrostatic gauges (such as the mercury column
The kenotometer is a particular type of hydrostatic gauge, typically used in power plants using steam turbines. The kenotometer measures the vacuum in the steam space of the condenser, that is, the exhaust of the last stage of the turbine.[41]
Mechanical or elastic gauges depend on a Bourdon tube, diaphragm, or capsule, usually made of metal, which will change shape in response to the pressure of the region in question. A variation on this idea is the capacitance manometer, in which the diaphragm makes up a part of a capacitor. A change in pressure leads to the flexure of the diaphragm, which results in a change in capacitance. These gauges are effective from 103 torr to 10−4 torr, and beyond.
Thermal conductivity gauges rely on the fact that the ability of a gas to conduct heat decreases with pressure. In this type of gauge, a wire filament is heated by running current through it. A
Uses

Vacuum is useful in a variety of processes and devices. Its first widespread use was in the
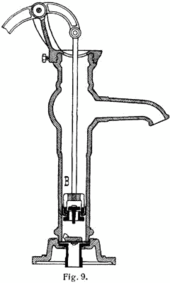
Vacuum-driven machines
Vacuums are commonly used to produce
Maintaining a vacuum in the
Outgassing
The most prevalent outgassing product in vacuum systems is water absorbed by chamber materials. It can be reduced by desiccating or baking the chamber, and removing absorbent materials. Outgassed water can condense in the oil of rotary vane pumps and reduce their net speed drastically if gas ballasting is not used. High vacuum systems must be clean and free of organic matter to minimize outgassing.
Ultra-high vacuum systems are usually baked, preferably under vacuum, to temporarily raise the vapour pressure of all outgassing materials and boil them off. Once the bulk of the outgassing materials are boiled off and evacuated, the system may be cooled to lower vapour pressures and minimize residual outgassing during actual operation. Some systems are cooled well below room temperature by liquid nitrogen to shut down residual outgassing and simultaneously cryopump the system.
Pumping and ambient air pressure

Fluids cannot generally be pulled, so a vacuum cannot be created by
To continue evacuating a chamber indefinitely without requiring infinite growth, a compartment of the vacuum can be repeatedly closed off, exhausted, and expanded again. This is the principle behind positive displacement pumps, like the manual water pump for example. Inside the pump, a mechanism expands a small sealed cavity to create a vacuum. Because of the pressure differential, some fluid from the chamber (or the well, in our example) is pushed into the pump's small cavity. The pump's cavity is then sealed from the chamber, opened to the atmosphere, and squeezed back to a minute size.

The above explanation is merely a simple introduction to vacuum pumping, and is not representative of the entire range of pumps in use. Many variations of the positive displacement pump have been developed, and many other pump designs rely on fundamentally different principles. Momentum transfer pumps, which bear some similarities to dynamic pumps used at higher pressures, can achieve much higher quality vacuums than positive displacement pumps. Entrapment pumps can capture gases in a solid or absorbed state, often with no moving parts, no seals and no vibration. None of these pumps are universal; each type has important performance limitations. They all share a difficulty in pumping low molecular weight gases, especially hydrogen, helium, and neon.
The lowest pressure that can be attained in a system is also dependent on many things other than the nature of the pumps. Multiple pumps may be connected in series, called stages, to achieve higher vacuums. The choice of seals, chamber geometry, materials, and pump-down procedures will all have an impact. Collectively, these are called vacuum technique. And sometimes, the final pressure is not the only relevant characteristic. Pumping systems differ in oil contamination, vibration, preferential pumping of certain gases, pump-down speeds, intermittent duty cycle, reliability, or tolerance to high leakage rates.
In
The lowest pressures currently achievable in laboratory are about 1×10−13 torrs (13 pPa).[43] However, pressures as low as 5×10−17 torrs (6.7 fPa) have been indirectly measured in a 4 K (−269.15 °C; −452.47 °F) cryogenic vacuum system.[4] This corresponds to ≈100 particles/cm3.
Effects on humans and animals

Humans and animals exposed to vacuum will lose
Animal experiments show that rapid and complete recovery is normal for exposures shorter than 90 seconds, while longer full-body exposures are fatal and resuscitation has never been successful.[47] A study by NASA on eight chimpanzees found all of them survived two and a half minute exposures to vacuum.[48] There is only a limited amount of data available from human accidents, but it is consistent with animal data. Limbs may be exposed for much longer if breathing is not impaired.[49] Robert Boyle was the first to show in 1660 that vacuum is lethal to small animals.
An experiment indicates that plants are able to survive in a low pressure environment (1.5 kPa) for about 30 minutes.[50][51]
Cold or oxygen-rich atmospheres can sustain life at pressures much lower than atmospheric, as long as the density of oxygen is similar to that of standard sea-level atmosphere. The colder air temperatures found at altitudes of up to 3 km generally compensate for the lower pressures there.
Rapid decompression can be much more dangerous than vacuum exposure itself. Even if the victim does not hold his or her breath, venting through the windpipe may be too slow to prevent the fatal rupture of the delicate alveoli of the lungs.[49] Eardrums and sinuses may be ruptured by rapid decompression, soft tissues may bruise and seep blood, and the stress of shock will accelerate oxygen consumption leading to hypoxia.[52] Injuries caused by rapid decompression are called barotrauma. A pressure drop of 13 kPa (100 Torr), which produces no symptoms if it is gradual, may be fatal if it occurs suddenly.[49]
Some
Examples
| Pressure (Pa or kPa) | Pressure (Torr, atm) | Mean free path | Molecules per cm3 | |
|---|---|---|---|---|
| Standard atmosphere, for comparison | 101.325 kPa | 760 torrs (1.00 atm) | 66 nm | 2.5×1019[54] |
| Intense hurricane
|
approx. 87 to 95 kPa | 650 to 710 | ||
| Vacuum cleaner | approximately 80 kPa | 600 | 70 nm | 1019 |
Condenser backpressure )
|
9 kPa | |||
liquid ring vacuum pump
|
approximately 3.2 kPa | 24 torrs (0.032 atm) | 1.75 μm | 1018 |
| Mars atmosphere | 1.155 kPa to 0.03 kPa (mean 0.6 kPa) | 8.66 to 0.23 torrs (0.01139 to 0.00030 atm) | ||
| freeze drying | 100 to 10 | 1 to 0.1 | 100 μm to 1 mm | 1016 to 1015 |
| Incandescent light bulb | 10 to 1 | 0.1 to 0.01 torrs (0.000132 to 1.3×10−5 atm) | 1 mm to 1 cm | 1015 to 1014 |
Thermos bottle
|
1 to 0.01 [1] | 1×10−2 to 1×10−4 torrs (1.316×10−5 to 1.3×10−7 atm) | 1 cm to 1 m | 1014 to 1012 |
| Earth thermosphere | 1 Pa to 1×10−7 | 10−2 to 10−9 | 1 cm to 100 km | 1014 to 107 |
| Vacuum tube | 1×10−5 to 1×10−8 | 10−7 to 10−10 | 1 to 1,000 km | 109 to 106 |
MBE chamber
|
1×10−7 to 1×10−9 | 10−9 to 10−11 | 100 to 10,000 km | 107 to 105 |
| Pressure on the Moon | approximately 1×10−9 | 10−11 | 10,000 km | 4×105[55] |
| Dense nebula | 10,000[1] | |||
Interplanetary space
|
11[1] | |||
| Interstellar space | 1[56] | |||
| Intergalactic space | 10−6[1] |
See also
- Decay of the vacuum (Pair production)
- Engine vacuum
- False vacuum
- Helium mass spectrometer – technical instrumentation to detect a vacuum leak
- Vacuum brazing
- Pneumatic tube – transport system using vacuum or pressure to move containers in tubes
- Rarefaction – reduction of a medium's density
- Suction – creation of a partial vacuum
- Theta vacuum – vacuum state of semi-classical pure-Yang Mills theories
- Vactrain
- Vacuum cementing – natural process of solidifying homogeneous "dust" in vacuum
- Vacuum column– controlling loose magnetic tape in early computer data recording tape drives
- Vacuum deposition – process of depositing atoms and molecules in a sub-atmospheric pressure environment
- Vacuum engineering
- vacuum systems
References
- ^ OCLC 55000526.[page needed]
- ISBN 978-0-07-707099-1.
- atm.
- ^ PMID 10042233.
- ^ atomic mass unitis 1.66×10−24 g, for roughly 40 atoms per cubic meter.
- ISBN 978-3-487-16076-4.
- ^ How to Make an Experimental Geissler Tube, Popular Science monthly, February 1919, Unnumbered page. Bonnier Corporation
- ^ "What words in the English language contain two u's in a row?". Oxford Dictionaries Online. Archived from the original on August 8, 2018. Retrieved 2011-10-23.
- ^ OCLC 48836264.
- ^ Druart, Therese-Anne (2016), "al-Farabi", in Zalta, Edward N. (ed.), Stanford Encyclopedia of Philosophy (Winter 2021 ed.), retrieved 2022-10-25
- ^ McGinnis, Jon (2022), "Arabic and Islamic Natural Philosophy and Natural Science", in Zalta, Edward N. (ed.), Stanford Encyclopedia of Philosophy (Spring 2022 ed.), retrieved 2022-08-11.
- ^ Dallal, Ahmad (2001–2002). "The Interplay of Science and Theology in the Fourteenth-century Kalam". From Medieval to Modern in the Islamic World, Sawyer Seminar at the University of Chicago. Archived from the original on 2012-02-10. Retrieved 2008-02-02.
- ).
- Donald Routledge Hill (1996), A History of Engineering in Classical and Medieval Times, Routledge, pp. 143, 150–152.
- LCCN 00058894.
- ISBN 978-0-521-22983-8.
- ^ OCLC 46600561.
- ^ "The World's Largest Barometer". Archived from the original on 2008-04-17. Retrieved 2008-04-30.
- ^ "Otto von Guericke | Prussian physicist, engineer, and philosopher | Britannica". www.britannica.com. Retrieved 2022-08-11.
- ^ Robert Hogarth Patterson, Essays in History and Art 10, 1862.
- .
- ^ ISBN 978-0-8194-4947-4.
- ISBN 978-0-444-52038-8.
- ISBN 978-0-521-55112-0.
...deals with the quantum vacuum where, in contrast to the classical vacuum, radiation has properties, in particular, fluctuations, with which one can associate physical effects.
- ISBN 978-0-316-01333-8.
- ISBN 978-0-691-12505-3.
- ^ "Speed of light in vacuum, c, c0". The NIST reference on constants, units, and uncertainty: Fundamental physical constants. NIST. Retrieved 2011-11-28.
- ISBN 978-81-224-1538-4.
- ^ "Electric constant, ε0". The NIST reference on constants, units, and uncertainty: Fundamental physical constants. NIST. Retrieved 2011-11-28.
- ^ "Magnetic constant, μ0". The NIST reference on constants, units, and uncertainty: Fundamental physical constants. NIST. Retrieved 2011-11-28.
- ^ "Characteristic impedance of vacuum, Z0". The NIST reference on constants, units, and uncertainty: Fundamental physical constants. Retrieved 2011-11-28.
- ISBN 978-0-444-53211-4.
- ISBN 978-0-486-40214-7.
- ISBN 978-3-642-22420-1.
- ISBN 978-3-540-74202-9.
The fundamental state of minimum energy, the vacuum, is not unique and there are a continuum of degenerate states that altogether respect the symmetry...
- ^ Squire, Tom (September 27, 2000). "U.S. Standard Atmosphere, 1976". Thermal Protection Systems Expert and Material Properties Database. Archived from the original on October 15, 2011. Retrieved 2011-10-23.
- ^ "Catalog of Earth Satellite Orbits". earthobservatory.nasa.gov. 2009-09-04. Retrieved 2019-01-28.
- S2CID 55324095. Archived from the original(PDF) on 2019-03-02. Retrieved 2019-07-21.
- OCLC 50287675.[page needed]
- ISBN 978-0-201-56947-6.
- ^ "Kenotometer Vacuum Gauge". Edmonton Power Historical Foundation. 22 November 2013. Retrieved 3 February 2014.
- ISBN 978-0-442-00522-1.
- doi:10.1116/1.575916.
- ^ Landis, Geoffrey (7 August 2007). "Human Exposure to Vacuum". geoffreylandis.com. Archived from the original on 21 July 2009. Retrieved 25 March 2006.
- hdl:2060/19730006364. NASA SP-3006.
- PMID 4872696.
- PMID 5972265.
- ^ Koestler, A. G. (November 1965). "The Effect on the Chimpanzee of Rapid Decompression to a near Vacuum" (PDF). NASA.
- ^ OCLC 18744945..
- hdl:2060/20130009997.
- PMID 11987308.
- ^ Czarnik, Tamarack R. (1999). "EBULLISM AT 1 MILLION FEET: Surviving Rapid/Explosive Decompression". unpublished review by Landis, Geoffrey A. geoffreylandis.
- S2CID 8566993.
- ^ Computed using "1976 Standard Atmosphere Properties" calculator. Retrieved 2012-01-28
- .
- ^ University of New Hampshire Experimental Space Plasma Group. "What is the Interstellar Medium". The Interstellar Medium, an online tutorial. Archived from the original on 2006-02-17. Retrieved 2006-03-15.
- Henning Genz (2001). Nothingness: The Science Of Empty Space. Da Capo Press. ISBN 978-0-7382-0610-3.
- Luciano Boi (2011). The Quantum Vacuum: A Scientific and Philosophical Concept, from Electrodynamics to String Theory and the Geometry of the Microscopic World. Johns Hopkins University Press. ISBN 978-1-4214-0247-5.
External links
- Leybold – Fundamentals of Vacuum Technology (PDF)
- VIDEO on the nature of vacuum by Canadian astrophysicist Doctor P
- The Foundations of Vacuum Coating Technology
- American Vacuum Society
- Journal of Vacuum Science and Technology A
- Journal of Vacuum Science and Technology B
- FAQ on explosive decompression and vacuum exposure.
- Discussion of the effects on humans of exposure to hard vacuum.
- Roberts, Mark D. (2000). "Vacuum Energy". High Energy Physics – Theory: hep–th/0012062. Bibcode:2000hep.th...12062R.
- Vacuum, Production of Space
- "Much Ado About Nothing" by Professor John D. Barrow, Gresham College
- Free pdf copy of The Structured Vacuum – thinking about nothing by ISBN 3-87144-889-3.


