Vasopressin
Physiological data | |
| Source tissues | Supraoptic nucleus; paraventricular nucleus of hypothalamus |
|---|---|
| Target tissues | System-wide |
| Receptors | V1A, V1B, V2, OXTR |
| Agonists | Felypressin, desmopressin |
| Antagonists | Diuretics |
| Metabolism | Predominantly in the liver and kidneys |
| Pharmacokinetic data | |
| Protein binding | 1% |
| Metabolism | Predominantly in the liver and kidneys |
| Elimination half-life | 10–20 minutes |
| Excretion | Urine |
| Identifiers | |
| |
JSmol) | |
| Density | 1.6±0.1 g/cm3 |
| |
| |
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) | |||||||||
| RefSeq (protein) | |||||||||
| Location (UCSC) | Chr 20: 3.08 – 3.08 Mb | Chr 2: 130.42 – 130.42 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin,[5] is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus,[6] and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.[7][8][9]
A third function is possible. Some AVP may be released directly into the
Vasopressin induces differentiation of stem cells into
It has a very short half-life, between 16 and 24 minutes.[9]
Physiology
Function
Vasopressin regulates the
AVP also may have a variety of neurological effects on the brain. It may influence pair-bonding in
A very similar substance, lysine vasopressin (LVP) or lypressin, has the same function in pigs and its synthetic version was used in human AVP deficiency, although it has been largely replaced by desmopressin.[14]
Kidney
Vasopressin has three main effects which are:
- Increasing the water permeability of distal convoluted tubule (DCT) and cortical collecting tubules (CCT), as well as outer and inner medullary collecting duct (OMCD & IMCD) in the kidney, thus allowing water reabsorption and excretion of more concentrated urine, i.e., V2 receptors. Vasopressin also increases the concentration of calcium in the collecting duct cells, by episodic release from intracellular stores. Vasopressin, acting through cAMP, also increases transcription of the aquaporin-2 gene, thus increasing the total number of aquaporin-2 molecules in collecting duct cells.[16]
- Increasing permeability of the inner medullary portion of the collecting duct to outer medullary collecting duct.
- Acute increase of collecting duct.[18]
Central nervous system
Vasopressin released within the brain may have several actions:
- Vasopressin is released into the brain in a circadian rhythm by neurons of the suprachiasmatic nucleus.[19]
- Vasopressin released from posterior pituitary is associated with nausea.[20]
- Recent evidence suggests that vasopressin may have analgesic effects. The analgesia effects of vasopressin were found to be dependent on both stress and sex.[21]
Regulation
Gene regulation
Vasopressin is regulated by
Many factors influence the secretion of vasopressin:
- Ethanol (alcohol) reduces the calcium-dependent secretion of AVP by blocking voltage-gated calcium channels in neurohypophyseal nerve terminals in rats.[24]
- Angiotensin II stimulates AVP secretion, in keeping with its general pressor and pro-volumic effects on the body.[25]
- Atrial natriuretic peptide inhibits AVP secretion, in part by inhibiting Angiotensin II-induced stimulation of AVP secretion.[25]
- Cortisol inhibits secretion of antidiuretic hormone.[26]
Production and secretion
The physiological stimulus for secretion of vasopressin is increased osmolality of the plasma, monitored by the hypothalamus. A decreased arterial blood volume, (such as can occur in cirrhosis, nephrosis, and heart failure), stimulates secretion, even in the face of decreased osmolality of the plasma: it supersedes osmolality, but with a milder effect. In other words, vasopressin secretion is also stimulated in the presence of hypoosmolality (hyponatremia) when the arterial blood volume is low by the unloading of
The AVP that is measured in peripheral blood is almost all derived from secretion from the
There are other sources of AVP, beyond the hypothalamic magnocellular neurons. For example, AVP is also synthesized by parvocellular neurosecretory neurons of the PVN, transported and released at the median eminence, from which it travels through the hypophyseal portal system to the anterior pituitary, where it stimulates corticotropic cells synergistically with CRH to produce ACTH (by itself it is a weak secretagogue).[28]
Vasopressin during surgery and anaesthesia
Vasopressin concentration is used to measure
Receptors
Types of AVP receptors and their actions:
| Type | Second messenger system | Locations | Actions | Agonists | Antagonists |
AVPR1A |
Phosphatidylinositol/calcium | Liver, kidney, peripheral vasculature, brain | Vasoconstriction, glycogen breakdown,[39] platelet aggregation, and release of factor VIII and von Willebrand factor; social recognition,[40] circadian tau[41] | Felypressin | |
AVPR1B or AVPR3 |
Phosphatidylinositol/calcium | Pituitary gland, brain | Adrenocorticotropic hormone secretion in response to stress;[42] social interpretation of olfactory cues[43] | ||
AVPR2 |
Adenylate cyclase/cAMP |
Basolateral membrane of the cells lining the collecting ducts of the kidneys (especially the cortical and outer medullary collecting ducts) |
Insertion of | AVP, desmopressin | "-vaptan" diuretics, i.e. tolvaptan |
Structure and relation to oxytocin

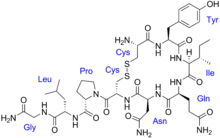
The vasopressins are
The structure of oxytocin is very similar to that of the vasopressins: It is also a nonapeptide with a disulfide bridge and its amino acid sequence differs at only two positions. The two genes are located on the same chromosome separated by a relatively small distance of less than 15,000 bases in most species. The magnocellular neurons that secrete vasopressin are adjacent to magnocellular neurons that secrete oxytocin, and are similar in many respects. The similarity of the two peptides can cause some cross-reactions: oxytocin has a slight antidiuretic function, and high levels of AVP can cause uterine contractions.[48][49]
Comparison of vasopressin and oxytocin neuropeptide families:
| Vertebrate Vasopressin Family | ||
|---|---|---|
| Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2 | Argipressin (AVP, ADH) |
Most mammals |
| Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Lys-Gly-NH2 | Lypressin (LVP) |
Pigs, hippos, warthogs, some marsupials |
| Cys-Phe-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2 | Phenypressin | Some marsupials |
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Arg-Gly-NH2 | Vasotocin† | Non-mammals |
| Vertebrate Oxytocin Family | ||
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2 | Oxytocin (OXT) | Most mammals, ratfish
|
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Pro-Gly-NH2 | Prol-Oxytocin | Some northern tree shrews
|
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Ile-Gly-NH2 | Mesotocin | Most marsupials, all birds, reptiles, amphibians, lungfishes, coelacanths |
| Cys-Tyr-Ile-Gln-Ser-Cys-Pro-Ile-Gly-NH2 | Seritocin | Frogs |
| Cys-Tyr-Ile-Ser-Asn-Cys-Pro-Ile-Gly-NH2 | Isotocin | Bony fishes
|
| Cys-Tyr-Ile-Ser-Asn-Cys-Pro-Gln-Gly-NH2 | Glumitocin | skates |
| Cys-Tyr-Ile-Asn/Gln-Asn-Cys-Pro-Leu/Val-Gly-NH2 | Various tocins | Sharks |
| Invertebrate VP/OT Superfamily | ||
| Cys-Leu-Ile-Thr-Asn-Cys-Pro-Arg-Gly-NH2 | Inotocin | Locust |
| Cys-Phe-Val-Arg-Asn-Cys-Pro-Thr-Gly-NH2 | Annetocin | Earthworm |
| Cys-Phe-Ile-Arg-Asn-Cys-Pro-Lys-Gly-NH2 | Lys-Connopressin | Geography & imperial sea hare, leech
|
| Cys-Ile-Ile-Arg-Asn-Cys-Pro-Arg-Gly-NH2 | Arg-Connopressin | Striped cone snail |
| Cys-Tyr-Phe-Arg-Asn-Cys-Pro-Ile-Gly-NH2 | Cephalotocin | Octopus |
| Cys-Phe-Trp-Thr-Ser-Cys-Pro-Ile-Gly-NH2 | Octopressin | Octopus |
| †Vasotocin is the evolutionary progenitor of all the vertebrate neurohypophysial hormones.[50] | ||
Medical use
Vasopressin is used to manage anti-diuretic hormone deficiency. Vasopressin is used to treat diabetes insipidus related to low levels of antidiuretic hormone. It is available as Pressyn.[51]
Vasopressin has off-label uses and is used in the treatment of vasodilatory shock, gastrointestinal bleeding, ventricular tachycardia and ventricular fibrillation.
Vasopressin agonists are used therapeutically in various conditions, and its long-acting synthetic analogue
Vasopressin infusions are also used as second line therapy for septic shock patients not responding to fluid resuscitation or infusions of catecholamines (e.g., dopamine or norepinephrine) to increase the blood pressure while sparing the use of catecholamines. These argipressins have much shorter elimination half-life (around 20 minutes) comparing to synthetic non-arginine vasopresines with much longer elimination half-life of many hours. Further, argipressins act on V1a, V1b, and V2 receptors which consequently lead to higher eGFR and lower vascular resistance in the lungs. A number of injectable arginine vasopressins are currently in clinical use in the United States and in Europe.
Pharmacokinetics
Vasopressin is administered through an
Side effects
The most common side effects during treatment with vasopressin are
Contraindications
The use of lysine vasopressin is contraindicated in the presence of hypersensitivity to beef or pork proteins, increased
Interactions
- alcohol - may lower the antidiuretic effect
- tricyclic antidepressants and fludrocortisonemay raise the diuretic effect
- lithium, demeclocycline, heparin or norepinephrine may lower the antidiuretic effect
- vasopressor effect may be higher with the concurrent use of ganglionic blocking medications[51]
Deficiency
Decreased AVP release (neurogenic — i.e. due to alcohol intoxication or tumour) or decreased renal sensitivity to AVP (nephrogenic, i.e. by mutation of V2 receptor or AQP) leads to diabetes insipidus, a condition featuring hypernatremia (increased blood sodium concentration), polyuria (excess urine production), and polydipsia (thirst).
Excess
History
Vasopressin was elucidated and synthesized for the first time by Vincent du Vigneaud.
Animal studies
Evidence for an effect of AVP on monogamy vs polygamy comes from experimental studies in several species, which indicate that the precise distribution of vasopressin and vasopressin receptors in the brain is associated with species-typical patterns of social behavior. In particular, there are consistent differences between monogamous species and polygamous species in the distribution of AVP receptors, and sometimes in the distribution of vasopressin-containing axons, even when closely related species are compared.[54]
Human studies
Vasopressin has shown
See also
- Syndrome of Inappropriate Antidiuretic Hormone secretion (SIADH)
- Oxytocin
- Vasopressin receptor
- Vasopressin receptor antagonists
- Copeptin
- Anterior Pituitary
- Hypothalamus
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000101200 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000037727 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ISBN 978-1-4160-6257-8.
- S2CID 35174328.
- ISBN 978-0-321-86158-0.
- ISBN 978-0-387-30348-2.
- ^ S2CID 36759747.
- PMID 20346754.
- PMID 26913138.
- PMID 30252325.
- S2CID 16210017.
- ^ Chapman IM, Professor of Medicine, Discipline of Medicine, University of Adelaide, Royal Adelaide Hospital. "Central Diabetes Insipidus". MSD. Merck & Co. Inc.
- OCLC 951680737.
- PMID 23584881.
- PMID 21686211.
- PMID 10073614.
- S2CID 2295415.
- PMID 27450627.
- S2CID 205434100.
- S2CID 14991100.
- S2CID 6916996.
- PMID 1941619.
- ^ PMID 21123762.
- ISBN 978-2-294-72233-2.
- ^ Garrahy A, Thompston CJ (2019). "General Principles, Diabetes, Metabolism, Obesity, Gastrointestinal Hormones, Aging, Endocrine Toxicology". Encyclopedia of Endocrine Diseases. 1 (2): 969–974.
- PMID 2830315.
- PMID 2360697.
- PMID 25130060.
- PMID 18758414.
- PMID 8390330.
- PMID 646620.
- S2CID 37668345.
- S2CID 43764321.
- PMID 14175989.
- ProQuest 223606053.
- PMID 15650630.
- ISBN 978-0-8153-4455-1.
- PMID 14647484.
- S2CID 29923520.
- PMID 17122081.
- S2CID 38444963.
- PMID 7545469.
- PMID 10880054.
- ISBN 978-1-4557-5942-2.
- ISBN 978-0-7506-0167-2.
- PMID 18057218.
- PMID 15280526.
- S2CID 12739464.
- ^ a b c d e "Vasopressin" (PDF). F.A. Davis Company. 2017. Retrieved 2017-03-13.[dead link]
- PMID 5101576.
- ^ PMID 17981159.
- S2CID 31960688.
- PMID 25853137.
- S2CID 3465601.
Further reading
- Rector FC, Brenner BM (2004). Brenner & Rector's the kidney (7th ed.). Philadelphia: Saunders. ISBN 978-0-7216-0164-9. Archived from the originalon 2016-03-03. Retrieved 2008-12-08.




