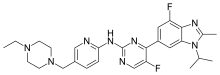
Abemaciclib
 | |
| Clinical data | |
|---|---|
| Pronunciation | /əˌbɛməˈsaɪklɪb/ ə-BEM-ə-SY-klib |
| Trade names | Verzenio, Verzenios, Ramiven, others |
| Other names | LY2835219 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 45% |
| Protein binding | 96.3% |
| Elimination half-life | 18.3 hrs |
| Excretion | 81% via feces, 3% via urine |
| Identifiers | |
| |
JSmol) | |
| |
| |
Abemaciclib, sold under the brand name Verzenio among others, is a
It was designated as a breakthrough therapy for breast cancer by the US Food and Drug Administration (FDA) in October 2015.[4]
In September 2017, it was approved for use in the United States by the FDA for the treatment of certain breast cancers.[5]
Medical uses
Since September 2017, abemaciclib has been approved in the US for "adults who have hormone receptor (HR)-positive,
In studies that compared fulvestrant plus abemaciclib to fulvestrant plus placebo in breast cancer patients, progression-free survival under abemaciclib therapy was 16.4 months on average, as compared to 9.3 months under the placebo arm.
Side effects
Side effects that occurred in 20% or more of patients in studies were diarrhea,
Interactions
As abemaciclib is mainly metabolized by the liver enzyme CYP3A4, inhibitors of this enzyme (such as ketoconazole) are expected to increase its blood plasma concentrations. Conversely, CYP3A4 inducers lower plasma concentrations of abemaciclib, as has been shown in a study with rifampicin.[7]
Pharmacology
Mechanism of action
Like the related drugs
Pharmacokinetics

After oral intake, absolute
Abemaciclib is excreted mainly via the feces (81%) and to a small extent via the urine (3%). Its
Clinical trials
Successful trials against pre-treated metastatic breast cancer were announced for Phase I in May 2014,[10] Phase II in December 2014,[11] and Phase III in February 2017.[12] Abemaciclib was approved by the FDA in September 2017 either in combination with fulvestrant or as a monotherapy for women with HR+, HER2- advanced or metastatic breast cancer that had progressed while receiving endocrine therapy.[13] Abemaciclib was approved for the adjuvant treatment of HR+, HER2-, node-positive adjuvant breast cancer at high risk of recurrence in March 2023.[14][15]
As of 2023, abemaciclib was involved in two Phase III clinical trials:
- The SARC041 study compares abemaciclib versus placebo in patients with advanced dedifferentiated liposarcoma.[16]
- The CYCLONE 3 study compares abemaciclib versus placebo in combination with abiraterone and prednisone in patients with high-risk, metastatic, hormone-sensitive prostate cancer.[17]
Abemaciclib is in Phase I and II clinical trials for head and neck squamous cell carcinoma,[18] biliary tract carcinoma,[19] brain tumors,[20][21][22] neurofibromatosis,[23] Kaposi sarcoma,[24] metastatic renal cell carcinoma,[25] and Mantle Cell lymphoma.[26]
Chemistry
Abemaciclib may be synthesized in a four step manner using a
References
- ^ "Verzenio (Eli Lilly Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 28 September 2022. Archived from the original on 18 March 2023. Retrieved 9 April 2023.
- ^ "Summary Basis of Decision (SBD) for Verzenio". Health Canada. 23 October 2014. Archived from the original on 31 May 2022. Retrieved 29 May 2022.
- PMID 26264704.
- ^ Digiulio S (8 October 2015). "FDA's Breakthrough Therapy Designation to Abemaciclib for Breast Cancer". Oncology Times. LWW Journals. Archived from the original on 4 June 2021. Retrieved 30 March 2016.
- ^ a b "FDA approves new treatment for certain advanced or metastatic breast cancers" (Press release). Food and Drug Administration. 28 September 2017. Archived from the original on 23 April 2019. Retrieved 29 September 2017.
- ^ Drugs.com: Abemaciclib Monograph. Accessed 2017-11-22.
- ^ a b c d e f "Highlights of Prescribing Information for Verzenio" (PDF). September 2017. Archived (PDF) from the original on 5 September 2021. Retrieved 22 November 2017.
- S2CID 12990398.
- PMID 32304130.
- ^ "LY2835219 Shows Strong Single-Agent Activity in Preliminary Study in Metastatic Breast Cancer". Archived from the original on 18 September 2015. Retrieved 8 January 2016.
- PMID 28533223.
- PMID 28580882.
- ^ Center for Drug Evaluation and Research (9 February 2019). "FDA approves abemaciclib for HR-positive, HER2-negative breast cancer". FDA.
- PMID 35084948.
- ^ Center for Drug Evaluation and Research (24 March 2023). "FDA D.I.S.C.O. Burst Edition: FDA approval of Verzenio (abemaciclib) with endocrine therapy for patients with HR-positive, HER2-negative, node-positive, early breast cancer". FDA.
- ^ "SARC041: Study of Abemaciclib Versus Placebo in Patients With Advanced Dedifferentiated Liposarcoma". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "A Study of Abemaciclib (LY2835219) With Abiraterone in Men With Prostate Cancer That Has Spread to Other Parts of the Body and is Expected to Respond to Hormonal Treatment (Metastatic Hormone-Sensitive Prostate Cancer) (CYCLONE 3)". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "Immune Modulation by Abemaciclib in HNSCC. (AIM Trial)". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "A Study to Evaluate Abemaciclib in Advanced Biliary Tract Carcinoma". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "Abemaciclib (LY2835219) in Patients With Recurrent Primary Brain Tumors". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "Abemaciclib in Children With DIPG or Recurrent/Refractory Solid Tumors (AflacST1501)". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "Abemaciclib w/Bevacizumab in Recurrent GBM Pts w/Loss of CDKN2A/B or Gain or Amplification of CDK4/6". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "Cyclin-Dependent Kinase (CDK)4/6 Inhibitor Abemaciclib for Neurofibromatosis Type I (NF1) Related Atypical Neurofibromas". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "Abemaciclib in Patients With HIV-associated and HIV-negative Kaposi Sarcoma". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "A Study of Abemaciclib in Combination With Sunitinib in Metastatic Renal Cell Carcinoma". www.clinicaltrials.gov. Retrieved 20 July 2023.
- ^ "Study of LY2835219 for Mantle Cell Lymphoma". www.clinicaltrials.gov. Retrieved 20 July 2023.
- .
