Xanthone

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Xanthen-9-one | |
| Other names
9-Oxoxanthene
Diphenyline ketone oxide | |
| Identifiers | |
3D model (
JSmol ) |
|
| 140443 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.001.816 |
| EC Number |
|
| 166003 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H8O2 | |
| Molar mass | 196.205 g·mol−1 |
| Appearance | white solid |
| Melting point | 174 °C (345 °F; 447 K) |
| Sl. sol. in hot water | |
| -108.1·10−6 cm3/mol | |
| Hazards | |
| GHS labelling:[1] | |

| |
| Danger | |
| H301 | |
| P264, P270, P301+P310, P321, P330, P405, P501 | |
| Related compounds | |
Related compounds
|
xanthene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Xanthone is an
molecular formula
C13H8O2. It is a white solid.
In 1939, xanthone was introduced as an
photocatalyst.[4]
Synthesis
Xanthone can be prepared by the heating of phenyl salicylate:[5]
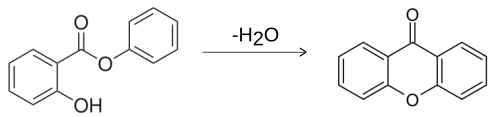
Six methods have been reported for synthesizing xanthone derivatives:[6]
- The Michael-Kostanecki method uses an equimolar mix of a polyphenol and an O-hydroxybenzoic acid, which are heated with a dehydrating agent.
- The Friedel-Crafts method has a benzophenone intermediate.
- The Robinson-Nishikawa method is a variant of the Hoesch synthesis but with low yields.
- The Asahina-Tanase method synthesizes some methoxylated xanthones, and xanthones with acid-sensitive substituents.
- The Tanase method is used to synthesize polyhydroxyxanthones.
- The diphenylether.
Xanthone derivatives
Xanthone forms the core of a variety of natural products, such as
pericarp of the mangosteen fruit (Garcinia mangostana) as well as in the bark and timber of Mesua thwaitesii.[9]
See also
References
- ^ "Xanthone". pubchem.ncbi.nlm.nih.gov.
- ^ Steiner, L. F. and S. A. Summerland. 1943. Xanthone as an ovicide and larvicide for the codling moth. Journal of Economic Entomology 36, 435-439.
- .
- PMID 27285582.
- .
- ISBN 978-0-08-045933-2.
- .
- ^ Williams, C.A; Harborne, J.B.; Colasante, M. (2000). "The pathway of chemical evolution in bearded iris species based on flavonoid and xanthone patterns" (PDF). Annali di Botanica. 58: 51–54. Retrieved 28 October 2015.
- .
