Xenon
 A xenon-filled discharge tube glowing light blue | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xenon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | colorless gas, exhibiting a blue glow when placed in an electric field | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Xe) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xenon in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 12.64 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 21.01[8] J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discovery and first isolation | William Ramsay and Morris Travers (1898) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of xenon | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Xenon is a
Xenon is used in
Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System.[26] Radioactive xenon-135 is produced by beta decay from iodine-135 (a product of nuclear fission), and is the most significant (and unwanted) neutron absorber in nuclear reactors.[27]
History
Xenon was discovered in England by the Scottish chemist William Ramsay and English chemist Morris Travers on July 12, 1898,[28] shortly after their discovery of the elements krypton and neon. They found xenon in the residue left over from evaporating components of liquid air.[29][30] Ramsay suggested the name xenon for this gas from the Greek word ξένον xénon, neuter singular form of ξένος xénos, meaning 'foreign(er)', 'strange(r)', or 'guest'.[31][32] In 1902, Ramsay estimated the proportion of xenon in the Earth's atmosphere to be one part in 20 million.[33]
During the 1930s, American engineer
In 1939, American physician Albert R. Behnke Jr. began exploring the causes of "drunkenness" in deep-sea divers. He tested the effects of varying the breathing mixtures on his subjects, and discovered that this caused the divers to perceive a change in depth. From his results, he deduced that xenon gas could serve as an anesthetic. Although Russian toxicologist Nikolay V. Lazarev apparently studied xenon anesthesia in 1941, the first published report confirming xenon anesthesia was in 1946 by American medical researcher John H. Lawrence, who experimented on mice. Xenon was first used as a surgical anesthetic in 1951 by American anesthesiologist Stuart C. Cullen, who successfully used it with two patients.[36]

Xenon and the other noble gases were for a long time considered to be completely chemically inert and not able to form compounds. However, while teaching at the University of British Columbia, Neil Bartlett discovered that the gas platinum hexafluoride (PtF6) was a powerful oxidizing agent that could oxidize oxygen gas (O2) to form dioxygenyl hexafluoroplatinate (O+
2[PtF
6]−
).[37] Since O2 (1165 kJ/mol) and xenon (1170 kJ/mol) have almost the same first ionization potential, Bartlett realized that platinum hexafluoride might also be able to oxidize xenon. On March 23, 1962, he mixed the two gases and produced the first known compound of a noble gas, xenon hexafluoroplatinate.[38][18]
Bartlett thought its composition to be Xe+[PtF6]−, but later work revealed that it was probably a mixture of various xenon-containing salts.[39][40][41] Since then, many other xenon compounds have been discovered,[42] in addition to some compounds of the noble gases argon, krypton, and radon, including argon fluorohydride (HArF),[43] krypton difluoride (KrF2),[44][45] and radon fluoride.[46] By 1971, more than 80 xenon compounds were known.[47][48]
In November 1989, IBM scientists demonstrated a technology capable of manipulating individual atoms. The program, called IBM in atoms, used a scanning tunneling microscope to arrange 35 individual xenon atoms on a substrate of chilled crystal of nickel to spell out the three letter company initialism. It was the first time atoms had been precisely positioned on a flat surface.[49]
Characteristics

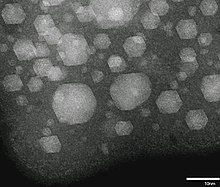
Xenon has atomic number 54; that is, its nucleus contains 54 protons. At standard temperature and pressure, pure xenon gas has a density of 5.894 kg/m3, about 4.5 times the density of the Earth's atmosphere at sea level, 1.217 kg/m3.[50] As a liquid, xenon has a density of up to 3.100 g/mL, with the density maximum occurring at the triple point.[51] Liquid xenon has a high polarizability due to its large atomic volume, and thus is an excellent solvent. It can dissolve hydrocarbons, biological molecules, and even water.[52] Under the same conditions, the density of solid xenon, 3.640 g/cm3, is greater than the average density of granite, 2.75 g/cm3.[51] Under gigapascals of pressure, xenon forms a metallic phase.[53]
Solid xenon changes from
(animated version
Liquid or solid xenon nanoparticles can be formed at room temperature by implanting Xe+ ions into a solid matrix. Many solids have lattice constants smaller than solid Xe. This results in compression of the implanted Xe to pressures that may be sufficient for its liquefaction or solidification.[56]
Xenon is a member of the zero-
In a gas-filled tube, xenon emits a blue or lavenderish glow when excited by electrical discharge. Xenon emits a band of emission lines that span the visual spectrum,[58] but the most intense lines occur in the region of blue light, producing the coloration.[59]
Occurrence and production
Xenon is a
Commercial
Xenon is obtained commercially as a by-product of the separation of air into oxygen and nitrogen.[61] After this separation, generally performed by fractional distillation in a double-column plant, the liquid oxygen produced will contain small quantities of krypton and xenon. By additional fractional distillation, the liquid oxygen may be enriched to contain 0.1–0.2% of a krypton/xenon mixture, which is extracted either by adsorption onto silica gel or by distillation. Finally, the krypton/xenon mixture may be separated into krypton and xenon by further distillation.[62][63]
Worldwide production of xenon in 1998 was estimated at 5,000–7,000 cubic metres (180,000–250,000 cu ft).[64] At a density of 5.894 grams per litre (0.0002129 lb/cu in) this is equivalent to roughly 30 to 40 tonnes (30 to 39 long tons; 33 to 44 short tons). Because of its scarcity, xenon is much more expensive than the lighter noble gases—approximate prices for the purchase of small quantities in Europe in 1999 were 10 €/L (=~€1.7/g) for xenon, 1 €/L (=~€0.27/g) for krypton, and 0.20 €/L (=~€0.22/g) for neon,[64] while the much more plentiful argon, which makes up over 1% by volume of earth's atmosphere, costs less than a cent per liter.
Solar System
Within the Solar System, the
Stellar
Unlike the lower-mass noble gases, the normal stellar nucleosynthesis process inside a star does not form xenon. Elements more massive than iron-56 consume energy through fusion, and the synthesis of xenon represents no energy gain for a star.[69] Instead, xenon is formed during supernova explosions during the r-process,[70] by the slow neutron-capture process (s-process) in red giant stars that have exhausted their core hydrogen and entered the asymptotic giant branch,[71] and from radioactive decay, for example by beta decay of extinct iodine-129 and spontaneous fission of thorium, uranium, and plutonium.[72]
Nuclear fission
Stable or extremely long lived isotopes of xenon are also produced in appreciable quantities in nuclear fission. Xenon-136 is produced when xenon-135 undergoes neutron capture before it can decay. The ratio of xenon-136 to xenon-135 (or its decay products) can give hints as to the power history of a given reactor and the absence of xenon-136 is a "fingerprint" for nuclear explosions, as xenon-135 is not produced directly but as a product of successive beta decays and thus it cannot absorb any neutrons in a nuclear explosion which occurs in fractions of a second.[75]
The stable isotope xenon-132 has a fission product yield of over 4% in the
U which means that stable or nearly stable xenon isotopes have a higher mass fraction in spent nuclear fuel (which is about 3% fission products) than it does in air. However, there is as of 2022 no commercial effort to extract xenon from spent fuel during nuclear reprocessing.[76][77]
Isotopes
Naturally occurring xenon is composed of seven
Nuclear spin
Nuclei of two of the stable
Because a 129Xe nucleus has a
From fission
Some radioactive isotopes of xenon (for example, 133Xe and 135Xe) are produced by
Isotope ratios of xenon produced in natural nuclear fission reactors at Oklo in Gabon reveal the reactor properties during chain reaction that took place about 2 billion years ago.[91]
Cosmic processes
Because xenon is a tracer for two parent isotopes, xenon isotope ratios in
Because the half-life of 129I is comparatively short on a cosmological time scale (16 million years), this demonstrated that only a short time had passed between the supernova and the time the meteorites had solidified and trapped the 129I. These two events (supernova and solidification of gas cloud) were inferred to have happened during the early history of the Solar System, because the 129I isotope was likely generated shortly before the Solar System was formed, seeding the solar gas cloud with isotopes from a second source. This supernova source may also have caused collapse of the solar gas cloud.[92][93]
In a similar way, xenon isotopic ratios such as 129Xe/130Xe and 136Xe/130Xe are a powerful tool for understanding planetary differentiation and early outgassing.[26] For example, the atmosphere of Mars shows a xenon abundance similar to that of Earth (0.08 parts per million[94]) but Mars shows a greater abundance of 129Xe than the Earth or the Sun. Since this isotope is generated by radioactive decay, the result may indicate that Mars lost most of its primordial atmosphere, possibly within the first 100 million years after the planet was formed.[95][96] In another example, excess 129Xe found in carbon dioxide well gases from New Mexico is believed to be from the decay of mantle-derived gases from soon after Earth's formation.[72][97]
Compounds
After Neil Bartlett's discovery in 1962 that xenon can form chemical compounds, a large number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the
Halides
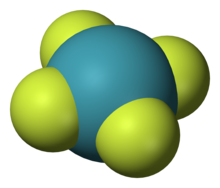

Three fluorides are known: XeF
2, XeF
4, and XeF
6. XeF is theorized to be unstable.[99] These are the starting points for the synthesis of almost all xenon compounds.
The solid, crystalline difluoride XeF
2 is formed when a mixture of fluorine and xenon gases is exposed to ultraviolet light.[100] The ultraviolet component of ordinary daylight is sufficient.[101] Long-term heating of XeF
2 at high temperatures under an NiF
2 catalyst yields XeF
6.[102] Pyrolysis of XeF
6 in the presence of NaF yields high-purity XeF
4.[103]
The xenon fluorides behave as both fluoride acceptors and fluoride donors, forming salts that contain such cations as XeF+
and Xe
2F+
3, and anions such as XeF−
5, XeF−
7, and XeF2−
8. The green, paramagnetic Xe+
2 is formed by the reduction of XeF
2 by xenon gas.[98]
XeF
2 also forms
Whereas the xenon fluorides are well characterized, the other halides are not. Xenon dichloride, formed by the high-frequency irradiation of a mixture of xenon, fluorine, and silicon or carbon tetrachloride,[104] is reported to be an endothermic, colorless, crystalline compound that decomposes into the elements at 80 °C. However, XeCl
2 may be merely a van der Waals molecule of weakly bound Xe atoms and Cl
2 molecules and not a real compound.[105] Theoretical calculations indicate that the linear molecule XeCl
2 is less stable than the van der Waals complex.[106] Xenon tetrachloride and xenon dibromide are more unstable that they cannot be synthesized by chemical reactions. They were created by radioactive decay of 129
ICl−
4 and 129
IBr−
2, respectively.[107][108]
Oxides and oxohalides
Three oxides of xenon are known: xenon trioxide (XeO
3) and xenon tetroxide (XeO
4), both of which are dangerously explosive and powerful oxidizing agents, and xenon dioxide (XeO2), which was reported in 2011 with a coordination number of four.[109] XeO2 forms when xenon tetrafluoride is poured over ice. Its crystal structure may allow it to replace silicon in silicate minerals.[110] The XeOO+ cation has been identified by infrared spectroscopy in solid argon.[111]
Xenon does not react with oxygen directly; the trioxide is formed by the hydrolysis of XeF
6:[112]
- XeF
6 + 3 H
2O → XeO
3 + 6 HF
XeO
3 is weakly acidic, dissolving in alkali to form unstable xenate salts containing the HXeO−
4 anion. These unstable salts easily disproportionate into xenon gas and perxenate salts, containing the XeO4−
6 anion.[113]
Barium perxenate, when treated with concentrated sulfuric acid, yields gaseous xenon tetroxide:[104]
- Ba
2XeO
6 + 2 H
2SO
4 → 2 BaSO
4 + 2 H
2O + XeO
4
To prevent decomposition, the xenon tetroxide thus formed is quickly cooled into a pale-yellow solid. It explodes above −35.9 °C into xenon and oxygen gas, but is otherwise stable.
A number of xenon oxyfluorides are known, including XeOF
2, XeOF
4, XeO
2F
2, and XeO
3F
2. XeOF
2 is formed by reacting OF
2 with xenon gas at low temperatures. It may also be obtained by partial hydrolysis of XeF
4. It disproportionates at −20 °C into XeF
2 and XeO
2F
2.[114] XeOF
4 is formed by the partial hydrolysis of XeF
6...[115]
- XeF
6 + H
2O → XeOF
4 + 2 HF
...or the reaction of XeF
6 with sodium perxenate, Na
4XeO
6. The latter reaction also produces a small amount of XeO
3F
2.
XeO
2F
2 is also formed by partial hydrolysis of XeF
6.[116]
- XeF
6 + 2 H
2O → XeO
2F
2 + 4 HF
XeOF
4 reacts with CsF to form the XeOF−
5 anion,[114][117] while XeOF3 reacts with the alkali metal fluorides KF, RbF and CsF to form the XeOF−
4 anion.[118]
Other compounds
Xenon can be directly bonded to a less electronegative element than fluorine or oxygen, particularly carbon.[119] Electron-withdrawing groups, such as groups with fluorine substitution, are necessary to stabilize these compounds.[113] Numerous such compounds have been characterized, including:[114][120]
- C
6F
5–Xe+
–N≡C–CH
3, where C6F5 is the pentafluorophenyl group. - [C
6F
5]
2Xe - C
6F
5–Xe–C≡N - C
6F
5–Xe–F - C
6F
5–Xe–Cl - C
2F
5–C≡C–Xe+ - [CH
3]
3C–C≡C–Xe+ - C
6F
5–XeF+
2 - (C
6F
5Xe)
2Cl+
Other compounds containing xenon bonded to a less electronegative element include F–Xe–N(SO
2F)
2 and F–Xe–BF
2. The latter is synthesized from dioxygenyl tetrafluoroborate, O
2BF
4, at −100 °C.[114][121]
An unusual ion containing xenon is the tetraxenonogold(II) cation, AuXe2+
4, which contains Xe–Au bonds.[122] This ion occurs in the compound AuXe
4(Sb
2F
11)
2, and is remarkable in having direct chemical bonds between two notoriously unreactive atoms, xenon and gold, with xenon acting as a transition metal ligand. A similar mercury complex (HgXe)(Sb3F17) (formulated as [HgXe2+][Sb2F11–][SbF6–]) is also known.[123]
The compound Xe
2Sb
2F
11 contains a Xe–Xe bond, the longest element-element bond known (308.71 pm = 3.0871 Å).[124]
In 1995, M. Räsänen and co-workers, scientists at the
Clathrates and excimers
In addition to compounds where xenon forms a
Xenon can also form endohedral fullerene compounds, where a xenon atom is trapped inside a fullerene molecule. The xenon atom trapped in the fullerene can be observed by 129Xe nuclear magnetic resonance (NMR) spectroscopy. Through the sensitive chemical shift of the xenon atom to its environment, chemical reactions on the fullerene molecule can be analyzed. These observations are not without caveat, however, because the xenon atom has an electronic influence on the reactivity of the fullerene.[134]
When xenon atoms are in the ground energy state, they repel each other and will not form a bond. When xenon atoms becomes energized, however, they can form an excimer (excited dimer) until the electrons return to the ground state. This entity is formed because the xenon atom tends to complete the outermost electronic shell by adding an electron from a neighboring xenon atom. The typical lifetime of a xenon excimer is 1–5 nanoseconds, and the decay releases photons with wavelengths of about 150 and 173 nm.[135][136] Xenon can also form excimers with other elements, such as the halogens bromine, chlorine, and fluorine.[137]
Applications
Although xenon is rare and relatively expensive to extract from the Earth's atmosphere, it has a number of applications.
Illumination and optics
Gas-discharge lamps
Xenon is used in light-emitting devices called xenon flash lamps, used in



Continuous, short-arc, high pressure
The individual cells in a plasma display contain a mixture of xenon and neon ionized with electrodes. The interaction of this plasma with the electrodes generates ultraviolet photons, which then excite the phosphor coating on the front of the display.[141][142]
Xenon is used as a "starter gas" in
Lasers
In 1962, a group of researchers at
Medical
| Clinical data | |
|---|---|
ECHA InfoCard | 100.028.338 |
Anesthesia
Xenon has been used as a
Xenon interacts with many different receptors and ion channels, and like many theoretically multi-modal inhalation anesthetics, these interactions are likely complementary. Xenon is a high-affinity glycine-site NMDA receptor antagonist.[149] However, xenon is different from certain other NMDA receptor antagonists in that it is not neurotoxic and it inhibits the neurotoxicity of ketamine and nitrous oxide (N2O), while actually producing neuroprotective effects.[150][151] Unlike ketamine and nitrous oxide, xenon does not stimulate a dopamine efflux in the nucleus accumbens.[152]
Like nitrous oxide and cyclopropane, xenon activates the two-pore domain potassium channel TREK-1. A related channel TASK-3 also implicated in the actions of inhalation anesthetics is insensitive to xenon.[153] Xenon inhibits nicotinic acetylcholine α4β2 receptors which contribute to spinally mediated analgesia.[154][155] Xenon is an effective inhibitor of plasma membrane Ca2+ ATPase. Xenon inhibits Ca2+ ATPase by binding to a hydrophobic pore within the enzyme and preventing the enzyme from assuming active conformations.[156]
Xenon is a competitive inhibitor of the serotonin 5-HT3 receptor. While neither anesthetic nor antinociceptive, this reduces anesthesia-emergent nausea and vomiting.[157]
Xenon has a
Neuroprotectant
Xenon induces robust cardioprotection and neuroprotection through a variety of mechanisms. Through its influence on Ca2+, K+, KATP\HIF, and NMDA antagonism, xenon is neuroprotective when administered before, during and after ischemic insults.[160][161] Xenon is a high affinity antagonist at the NMDA receptor glycine site.[149] Xenon is cardioprotective in ischemia-reperfusion conditions by inducing pharmacologic non-ischemic preconditioning. Xenon is cardioprotective by activating PKC-epsilon and downstream p38-MAPK.[162] Xenon mimics neuronal ischemic preconditioning by activating ATP sensitive potassium channels.[163] Xenon allosterically reduces ATP mediated channel activation inhibition independently of the sulfonylurea receptor1 subunit, increasing KATP open-channel time and frequency.[164]
Sports doping
Inhaling a xenon/oxygen mixture activates production of the
Imaging
Xenon, particularly hyperpolarized 129Xe, is a useful contrast agent for
Xenon-129 is currently being used as a visualization agent in MRI scans. When a patient inhales hyperpolarized xenon-129 ventilation and gas exchange in the lungs can be imaged and quantified. Unlike xenon-133, xenon-129 is non-ionizing and is safe to be inhaled with no adverse effects.[176]
Surgery
The xenon chloride excimer laser has certain dermatological uses.[177]
NMR spectroscopy
Because of the xenon atom's large, flexible outer electron shell, the NMR spectrum changes in response to surrounding conditions and can be used to monitor the surrounding chemical circumstances. For instance, xenon dissolved in water, xenon dissolved in hydrophobic solvent, and xenon associated with certain proteins can be distinguished by NMR.[178][179]
Hyperpolarized xenon can be used by
Other
In nuclear energy studies, xenon is used in bubble chambers,[182] probes, and in other areas where a high molecular weight and inert chemistry is desirable. A by-product of nuclear weapon testing is the release of radioactive xenon-133 and xenon-135. These isotopes are monitored to ensure compliance with nuclear test ban treaties,[183] and to confirm nuclear tests by states such as North Korea.[184]

Liquid xenon is used in
Xenon is the preferred
Chemically, the
Precautions
| Hazards | |
|---|---|
| NFPA 704 (fire diamond) | |
Xenon gas can be safely kept in normal sealed glass or metal containers at
The speed of sound in xenon gas (169 m/s) is less than that in air[195] because the average velocity of the heavy xenon atoms is less than that of nitrogen and oxygen molecules in air. Hence, xenon vibrates more slowly in the vocal cords when exhaled and produces lowered voice tones (low-frequency-enhanced sounds, but the fundamental frequency or pitch does not change), an effect opposite to the high-toned voice produced in helium. Specifically, when the vocal tract is filled with xenon gas, its natural resonant frequency becomes lower than when it is filled with air. Thus, the low frequencies of the sound wave produced by the same direct vibration of the vocal cords would be enhanced, resulting in a change of the timbre of the sound amplified by the vocal tract. Like helium, xenon does not satisfy the body's need for oxygen, and it is both a simple asphyxiant and an anesthetic more powerful than nitrous oxide; consequently, and because xenon is expensive, many universities have prohibited the voice stunt as a general chemistry demonstration.[citation needed] The gas sulfur hexafluoride is similar to xenon in molecular weight (146 versus 131), less expensive, and though an asphyxiant, not toxic or anesthetic; it is often substituted in these demonstrations.[196]
Dense gases such as xenon and sulfur hexafluoride can be breathed safely when mixed with at least 20% oxygen. Xenon at 80% concentration along with 20% oxygen rapidly produces the unconsciousness of general anesthesia. Breathing mixes gases of different densities very effectively and rapidly so that heavier gases are purged along with the oxygen, and do not accumulate at the bottom of the lungs.[197] There is, however, a danger associated with any heavy gas in large quantities: it may sit invisibly in a container, and a person who enters an area filled with an odorless, colorless gas may be asphyxiated without warning. Xenon is rarely used in large enough quantities for this to be a concern, though the potential for danger exists any time a tank or container of xenon is kept in an unventilated space.[198]
Water-soluble xenon compounds such as
See also
- Buoyant levitation
- Noble gases
- Penning mixture
Notes
- ^ Mass fraction calculated from the average mass of an atom in the Solar System of about 1.29 atomic mass units.
References
- ^ "xenon". Oxford English Dictionary. Vol. 20 (2nd ed.). Oxford University Press. 1989.
- ^ "Xenon". Dictionary.com Unabridged. 2010. Retrieved May 6, 2010.
- ^ "Standard Atomic Weights: Xenon". CIAAW. 1999.
- ISSN 1365-3075.
- ^ ISBN 978-1-62708-155-9.
- ^ "Xenon". Gas Encyclopedia. Air Liquide. 2009.
- ^ ISBN 1-4398-5511-0.
- ISBN 0-471-23896-1.
- ISBN 0-8493-0486-5.
- ISBN 0-8493-0464-4.
- .
- .
- .
- .
- ^ "Xenon". Columbia Electronic Encyclopedia (6th ed.). Columbia University Press. 2007. Retrieved 2007-10-23.
- ^ a b Husted, Robert; Boorman, Mollie (December 15, 2003). "Xenon". Los Alamos National Laboratory, Chemical Division. Retrieved 2007-09-26.
- )
- ^ .
- ^ ISBN 0-7432-2619-4.
- ^ a b
Mellor, David (2000). Sound Person's Guide to Video. ISBN 0-240-51595-1.
- PMID 15728132.
- ^ a b Basov, N. G.; Danilychev, V. A.; Popov, Yu. M. (1971). "Stimulated Emission in the Vacuum Ultraviolet Region". Soviet Journal of Quantum Electronics. 1 (1): 18–22. .
- ^ a b
Toyserkani, E.; Khajepour, A.; Corbin, S. (2004). Laser Cladding. CRC Press. p. 48. ISBN 0-8493-2172-7.
- . Retrieved 2007-10-08.
- ^ a b Saccoccia, G.; del Amo, J. G.; Estublier, D. (August 31, 2006). "Ion engine gets SMART-1 to the Moon". ESA. Retrieved 2007-10-01.
- ^ a b
Kaneoka, Ichiro (1998). "Xenon's Inside Story". Science. 280 (5365): 851–852. S2CID 128502357.
- ^ a b
Stacey, Weston M. (2007). Nuclear Reactor Physics. Wiley-VCH. p. 213. ISBN 978-3-527-40679-1.
- ^ Ramsay, Sir William (July 12, 1898). "Nobel Lecture – The Rare Gases of the Atmosphere". Nobel prize. Nobel Media AB. Retrieved 15 November 2015.
- ^ Ramsay, W.; Travers, M. W. (1898). "On the extraction from air of the companions of argon, and neon". Report of the Meeting of the British Association for the Advancement of Science: 828.
- ^ Gagnon, Steve. "It's Elemental – Xenon". Thomas Jefferson National Accelerator Facility. Retrieved 2007-06-16.
- Dodd, Mead and Company. p. 906.
- ISBN 0-87779-603-3.
- S2CID 97151557.
- ^ "History". Millisecond Cinematography. Archived from the original on 2006-08-22. Retrieved 2007-11-07.
- ^ Paschotta, Rüdiger (November 1, 2007). "Lamp-pumped lasers". Encyclopedia of Laser Physics and Technology. RP Photonics. Retrieved 2007-11-07.
- ^
Marx, Thomas; Schmidt, Michael; Schirmer, Uwe; Reinelt, Helmut (2000). "Xenon anesthesia" (PDF). Journal of the Royal Society of Medicine. 93 (10): 513–7. PMID 11064688. Retrieved 2007-10-02.
- ^
Bartlett, Neil; Lohmann, D. H. (1962). "Dioxygenyl hexafluoroplatinate (V), O+
2[PtF
6]−
". Proceedings of the Chemical Society (3). London: Chemical Society: 115. . - ^ Bartlett, N. (1962). "Xenon hexafluoroplatinate (V) Xe+[PtF6]−". Proceedings of the Chemical Society (6). London: .
- ^ Graham, L.; Graudejus, O.; Jha N.K.; Bartlett, N. (2000). "Concerning the nature of XePtF6". Coordination Chemistry Reviews. 197 (1): 321–34. .
- ISBN 3-11-012641-9.
- ^ Steel, Joanna (2007). "Biography of Neil Bartlett". College of Chemistry, University of California, Berkeley. Archived from the original on September 23, 2009. Retrieved 2007-10-25.
- ^ Bartlett, Neil (2003-09-09). "The Noble Gases". Chemical & Engineering News. 81 (36). American Chemical Society: 32–34. . Retrieved 2007-10-01.
- S2CID 4382128.
- ^
Lynch, C. T.; Summitt, R.; Sliker, A. (1980). CRC Handbook of Materials Science. ISBN 0-87819-231-X.
- S2CID 44475654.
- ^ Paul R. Fields; Lawrence Stein & Moshe H. Zirin (1962). "Radon Fluoride". .
- ^ "Xenon". Periodic Table Online. CRC Press. Archived from the original on April 10, 2007. Retrieved 2007-10-08.
- . Retrieved 2007-10-16.
- ^ Browne, Malcolm W. (April 5, 1990) "2 Researchers Spell 'I.B.M.,' Atom by Atom". New York Times
- ^ Williams, David R. (April 19, 2007). "Earth Fact Sheet". NASA. Retrieved 2007-10-04.
- ^ a b
Aprile, Elena; Bolotnikov, Aleksey E.; Doke, Tadayoshi (2006). Noble Gas Detectors. ISBN 3-527-60963-6.
- S2CID 4237285.
- ^ Caldwell, W. A.; Nguyen, J.; Pfrommer, B.; Louie, S.; .
- ^ Fontes, E. "Golden Anniversary for Founder of High-pressure Program at CHESS". Cornell University. Retrieved 2009-05-30.
- ^
S2CID 19937739.
- ^
Iakoubovskii, Konstantin; Mitsuishi, Kazutaka; Furuya, Kazuo (2008). "Structure and pressure inside Xe nanoparticles embedded in Al". Physical Review B. 78 (6): 064105. S2CID 29156048.
- ^ Bader, Richard F. W. "An Introduction to the Electronic Structure of Atoms and Molecules". McMaster University. Retrieved 2007-09-27.
- ^ Talbot, John. "Spectra of Gas Discharges". Rheinisch-Westfälische Technische Hochschule Aachen. Archived from the original on July 18, 2007. Retrieved 2006-08-10.
- ^
Watts, William Marshall (1904). An Introduction to the Study of Spectrum Analysis. London: Longmans, Green, and Co.
- ^
Hwang, Shuen-Cheng; Robert D. Lein; Daniel A. Morgan (2005). "Noble Gases". Kirk-Othmer Encyclopedia of Chemical Technology (5th ed.). ISBN 0-471-48511-X.
- ^ Lebedev, P. K.; Pryanichnikov, V. I. (1993). "Present and future production of xenon and krypton in the former USSR region and some physical properties of these gases" (PDF). Nuclear Instruments and Methods in Physics Research A. 327 (1): 222–226. .
- ^
Kerry, Frank G. (2007). Industrial Gas Handbook: Gas Separation and Purification. CRC Press. pp. 101–103. ISBN 978-0-8493-9005-0.
- ^ "Xenon – Xe". CFC StarTec LLC. August 10, 1998. Archived from the original on 2020-06-12. Retrieved 2007-09-07.
- ^ ISBN 3-527-20165-3.
- ISBN 0-691-01147-8.
- ^ Mahaffy, P. R.; Niemann, H. B.; Alpert, A.; Atreya, S. K.; Demick, J.; Donahue, T. M.; Harpold, D. N.; Owen, T. C. (2000). "Noble gas abundance and isotope ratios in the atmosphere of Jupiter from the Galileo Probe Mass Spectrometer". Journal of Geophysical Research. 105 (E6): 15061–72. .
- ^
Owen, Tobias; Mahaffy, Paul; Niemann, H. B.; Atreya, Sushil; Donahue, Thomas; Bar-Nun, Akiva; de Pater, Imke (1999). "A low-temperature origin for the planetesimals that formed Jupiter" (PDF). Nature. 402 (6759): 269–70. S2CID 4426771.
- S2CID 31226092.
- ISBN 0-226-10953-4.
- ^
Heymann, D.; Dziczkaniec, M. (March 19–23, 1979). Xenon from intermediate zones of supernovae. Proceedings 10th Lunar and Planetary Science Conference. Houston, Texas: Pergamon Press, Inc. pp. 1943–1959. Bibcode:1979LPSC...10.1943H.
- ^
Beer, H.; Kaeppeler, F.; Reffo, G.; Venturini, G. (November 1983). "Neutron capture cross-sections of stable xenon isotopes and their application in stellar nucleosynthesis". Astrophysics and Space Science. 97 (1): 95–119. S2CID 123139238.
- ^ a b c Caldwell, Eric (January 2004). "Periodic Table – Xenon". Resources on Isotopes. USGS. Retrieved 2007-10-08.
- ^ ""Xenon Poisoning" or Neutron Absorption in Reactors".
- ^ "Chernobyl Appendix 1: Sequence of Events – World Nuclear Association".
- .
- ^ "Novel gas-capture approach advances nuclear fuel management". 24 July 2020.
- ^ "What's in Spent Nuclear Fuel? (After 20 yrs) – Energy from Thorium". 22 June 2010.
- ^
Barabash, A. S. (2002). "Average (Recommended) Half-Life Values for Two-Neutrino Double-Beta Decay". Czechoslovak Journal of Physics. 52 (4): 567–573. S2CID 15146959.
- S2CID 129948831.
- S2CID 40334443.
- ^
Otten, Ernst W. (2004). "Take a breath of polarized noble gas". Europhysics News. 35 (1): 16–20. S2CID 51224754.
- ^
Ruset, I. C.; Ketel, S.; Hersman, F. W. (2006). "Optical Pumping System Design for Large Production of Hyperpolarized 129Xe". Physical Review Letters. 96 (5): 053002. PMID 16486926.
- ^
Wolber, J.; Cherubini, A.; Leach, M. O.; Bifone, A. (2000). "On the oxygenation-dependent 129Xe t1 in blood". S2CID 94795359.
- PMID 11909399.
- ^
von Schulthess, Gustav Konrad; Smith, Hans-Jørgen; Pettersson, Holger; Allison, David John (1998). The Encyclopaedia of Medical Imaging. Taylor & Francis. p. 194. ISBN 1-901865-13-4.
- ^ Warren, W. W.; Norberg, R. E. (1966). "Nuclear Quadrupole Relaxation and Chemical Shift of Xe131 in Liquid and Solid Xenon". Physical Review. 148 (1): 402–412. .
- ^ Staff. "Hanford Becomes Operational". The Manhattan Project: An Interactive History. U.S. Department of Energy. Archived from the original on 2009-12-10. Retrieved 2007-10-10.
- ^
Pfeffer, Jeremy I.; Nir, Shlomo (2000). Modern Physics: An Introductory Text. ISBN 1-86094-250-4.
- ^
Laws, Edwards A. (2000). Aquatic Pollution: An Introductory Text. John Wiley and Sons. p. 505. ISBN 0-471-34875-9.
- ^ Staff (April 9, 1979). "A Nuclear Nightmare". Time. Archived from the original on October 12, 2007. Retrieved 2007-10-09.
- PMID 15525157.
- ^ a b
Clayton, Donald D. (1983). Principles of Stellar Evolution and Nucleosynthesis (2nd ed.). University of Chicago Press. p. 75. ISBN 0-226-10953-4.
- ^ a b
Bolt, B. A.; Packard, R. E.; Price, P. B. (2007). "John H. Reynolds, Physics: Berkeley". The University of California, Berkeley. Retrieved 2007-10-01.
- ^ Williams, David R. (September 1, 2004). "Mars Fact Sheet". NASA. Archived from the original on 2010-06-12. Retrieved 2007-10-10.
- ^ Schilling, James. "Why is the Martian atmosphere so thin and mainly carbon dioxide?". Mars Global Circulation Model Group. Archived from the original on 2010-05-28. Retrieved 2007-10-10.
- ^ Zahnle, Kevin J. (1993). "Xenological constraints on the impact erosion of the early Martian atmosphere". .
- ^
Boulos, M. S.; Manuel, O.K. (1971). "The xenon record of extinct radioactivities in the Earth". S2CID 28159702.
- ^ ISBN 0-85404-690-9.
- .
- .
- .
- ^ .
- .
- ^ ISBN 3-11-011451-8.
- .
- .
- ISBN 978-1-4832-8060-8.
- ISBN 978-1-4831-5736-8.
- PMID 21341650.
- doi:10.1038/471138d.
- PMID 16492012.
- ISBN 0-12-023646-X.
- ^ ISBN 0-85404-617-8.
- ^ ISBN 0-7487-6420-8.
- S2CID 42752536.
- ISBN 978-81-7450-648-1.
- .
- .
- ^
Holloway, John H.; Hope, Eric G. (1998). Advances in Inorganic Chemistry. Contributor A. G. Sykes. Academic Press. pp. 61–90. ISBN 0-12-023646-X.
- ^ Frohn, H.; Theißen, Michael (2004). "C6F5XeF, a versatile starting material in xenon–carbon chemistry". Journal of Fluorine Chemistry. 125 (6): 981–988. .
- ^ Goetschel, Charles T.; Loos, Karl R. (1972). "Reaction of xenon with dioxygenyl tetrafluoroborate. Preparation of FXe-BF2". Journal of the American Chemical Society. 94 (9): 3018–3021. .
- ^
Li, Wai-Kee; Zhou, Gong-Du; Mak, Thomas C. W. (2008). Gong-Du Zhou; Thomas C. W. Mak (eds.). Advanced Structural Inorganic Chemistry. ISBN 978-0-19-921694-9.
- PMID 14502720.
- ^
Li, Wai-Kee; Zhou, Gong-Du; Mak, Thomas C. W. (2008). Advanced Structural Inorganic Chemistry. Oxford University Press. p. 674. ISBN 978-0-19-921694-9.
- ^
Gerber, R. B. (2004). "Formation of novel rare-gas molecules in low-temperature matrices". Annual Review of Physical Chemistry. 55 (1): 55–78. PMID 15117247.
- PMID 18407641.
- .
- ^
ISBN 981-02-2940-2.
- ISBN 0-85404-617-8.
- .
- .
- ^
McKay, C. P.; Hand, K. P.; Doran, P. T.; Andersen, D. T.; Priscu, J. C. (2003). "Clathrate formation and the fate of noble and biologically useful gases in Lake Vostok, Antarctica". Geophysical Research Letters. 30 (13): 35. S2CID 20136021.
- S2CID 97577041.
- ^
Frunzi, Michael; Cross, R. James; Saunders, Martin (2007). "Effect of Xenon on Fullerene Reactions". Journal of the American Chemical Society. 129 (43): 13343–6. PMID 17924634.
- ^
Silfvast, William Thomas (2004). Laser Fundamentals. ISBN 0-521-83345-0.
- ^
Webster, John G. (1998). The Measurement, Instrumentation, and Sensors Handbook. Springer. ISBN 3-540-64830-5.
- ^
McGhee, Charles; Taylor, Hugh R.; Gartry, David S.; Trokel, Stephen L. (1997). Excimer Lasers in Ophthalmology. Informa Health Care. ISBN 1-85317-253-7.
- ^ Staff (2007). "Xenon Applications". Praxair Technology. Archived from the original on 2013-03-22. Retrieved 2007-10-04.
- ^
Baltás, E.; Csoma, Z.; Bodai, L.; Ignácz, F.; Dobozy, A.; Kemény, L. (2003). "A xenon-iodine electric discharge bactericidal lamp". Technical Physics Letters. 29 (10): 871–72. S2CID 122651818.
- ^ Skeldon, M. D.; Saager, R.; Okishev, A.; Seka, W. (1997). "Thermal distortions in laser-diode- and flash-lamp-pumped Nd:YLF laser rods" (PDF). LLE Review. 71: 137–44. Archived from the original (PDF) on October 16, 2003. Retrieved 2007-02-04.
- ^ Anonymous. "The plasma behind the plasma TV screen". Plasma TV Science. Archived from the original on October 15, 2007. Retrieved 2007-10-14.
- ^ Marin, Rick (March 21, 2001). "Plasma TV: That New Object Of Desire". The New York Times. Retrieved 2009-04-03.
- ISBN 0-262-23048-8.
- ^ Patel, C. K. N.; Bennett Jr., W. R.; Faust, W. L.; McFarlane, R. A. (August 1, 1962). "Infrared spectroscopy using stimulated emission techniques". Physical Review Letters. 9 (3): 102–4. .
- ^ Patel, C. K. N.; Faust, W. L.; McFarlane, R. A. (December 1, 1962). "High gain gaseous (Xe-He) optical masers". Applied Physics Letters. 1 (4): 84–85. .
- ^ Bennett, Jr., W. R. (1962). "Gaseous optical masers". Applied Optics. 1 (S1): 24–61. .
- ^ "Laser Output". University of Waterloo. Archived from the original on 2011-07-06. Retrieved 2007-10-07.
- PMID 27530275.
- ^ PMID 20124979.
- PMID 12393773.
- S2CID 15167421.
- S2CID 1882085.
- S2CID 7762447.
- S2CID 4684919.
- S2CID 53151557.
- PMID 7499320.
- S2CID 6705116.
- PMID 12878613.
- S2CID 19119058.
- S2CID 25266308.
- PMID 16373643.
- PMID 15644876.
- PMID 19352153.
- PMID 20179498.
- ^ "Breathe it in". The Economist. 8 February 2014.
- ^ "WADA amends Section S.2.1 of 2014 Prohibited List". 31 August 2014. Archived from the original on 27 April 2021. Retrieved 1 September 2014.
- S2CID 55832101.
- ^
Van Der Wall, Ernst (1992). What's New in Cardiac Imaging?: SPECT, PET, and MRI. Springer. ISBN 0-7923-1615-0.
- ^ Frank, John (1999). "Introduction to imaging: The chest". Student BMJ. 12: 1–44. Retrieved 2008-06-04.
- ^ Chandak, Puneet K. (July 20, 1995). "Brain SPECT: Xenon-133". Brigham RAD. Archived from the original on January 4, 2012. Retrieved 2008-06-04.
- ^
Albert, M. S.; Balamore, D. (1998). "Development of hyperpolarized noble gas MRI". Nuclear Instruments and Methods in Physics Research A. 402 (2–3): 441–53. PMID 11543065.
- ^ Irion, Robert (March 23, 1999). "Head Full of Xenon?". Science News. Archived from the original on January 17, 2004. Retrieved 2007-10-08.
- ^
Wolber, J.; Rowland, I. J.; Leach, M. O.; Bifone, A. (1998). "Intravascular delivery of hyperpolarized 129Xenon for in vivo MRI". Applied Magnetic Resonance. 15 (3–4): 343–52. S2CID 100913538.
- ^
Driehuys, B.; Möller, H.E.; Cleveland, Z.I.; Pollaro, J.; Hedlund, L.W. (2009). "Pulmonary perfusion and xenon gas exchange in rats: MR imaging with intravenous injection of hyperpolarized 129Xe". Radiology. 252 (2): 386–93. PMID 19703880.
- ^
Cleveland, Z.I.; Möller, H.E.; Hedlund, L.W.; Driehuys, B. (2009). "Continuously infusing hyperpolarized 129Xe into flowing aqueous solutions using hydrophobic gas exchange membranes". The Journal of Physical Chemistry. 113 (37): 12489–99. PMID 19702286.
- PMID 33632417.
- S2CID 20156819.
- ^
Luhmer, M.; Dejaegere, A.; Reisse, J. (1989). "Interpretation of the solvent effect on the screening constant of Xe-129". Magnetic Resonance in Chemistry. 27 (10): 950–52. S2CID 95432492.
- ^
Rubin, Seth M.; Spence, Megan M.; Goodson, Boyd M.; Wemmer, David E.; Pines, Alexander (August 15, 2000). "Evidence of nonspecific surface interactions between laser-polarized xenon and myoglobin in solution". PMID 10931956.
- ^ Raftery, Daniel; MacNamara, Ernesto; Fisher, Gregory; Rice, Charles V.; Smith, Jay (1997). "Optical Pumping and Magic Angle Spinning: Sensitivity and Resolution Enhancement for Surface NMR Obtained with Laser-Polarized Xenon". Journal of the American Chemical Society. 119 (37): 8746–47. .
- ^
Gaede, H. C.; Song, Y. -Q.; Taylor, R. E.; Munson, E. J.; Reimer, J. A.; Pines, A. (1995). "High-field cross polarization NMR from laser-polarized xenon to surface nuclei". Applied Magnetic Resonance. 8 (3–4): 373–84. S2CID 34971961.
- ^
Galison, Peter Louis (1997). Image and Logic: A Material Culture of Microphysics. University of Chicago Press. p. 339. ISBN 0-226-27917-0.
- ^
Fontaine, J.-P.; Pointurier, F.; Blanchard, X.; Taffary, T. (2004). "Atmospheric xenon radioactive isotope monitoring". Journal of Environmental Radioactivity. 72 (1–2): 129–35. PMID 15162864.
- ^ Garwin, Richard L.; von Hippel Frank N. (November 2006). "A Technical Analysis: Deconstructing North Korea's October 9 Nuclear Test". Arms Control Today. 38 (9). Arms Control Association. Retrieved 2009-03-26.
- ^ Gallucci, G. (2009). "The MEG liquid xenon calorimeter". Journal of Physics: Conference Series. 160 (1): 012011. .
- ^ Zona, Kathleen (March 17, 2006). "Innovative Engines: Glenn Ion Propulsion Research Tames the Challenges of 21st century Space Travel". NASA. Archived from the original on September 15, 2007. Retrieved 2007-10-04.
- ^ "Dawn Launch: Mission to Vesta and Ceres" (PDF). NASA. Retrieved 2007-10-01.
- ^
Brazzle, J. D.; Dokmeci, M. R.; Mastrangelo, C. H. (July 28 – August 1, 1975). Modeling and Characterization of Sacrificial Polysilicon Etching Using Vapor-Phase Xenon Difluoride. Proceedings 17th IEEE International Conference on Micro Electro Mechanical Systems (MEMS). Maastricht, Netherlands: IEEE. pp. 737–40. ISBN 978-0-7803-8265-7.
- ^ Staff (2007). "Neil Bartlett and the Reactive Noble Gases". American Chemical Society. Retrieved June 5, 2012.
- ^ Staff (December 21, 2004). "Protein Crystallography: Xenon and Krypton Derivatives for Phasing". Daresbury Laboratory, PX. Archived from the original on 2005-03-16. Retrieved 2007-10-01.
- ^
ISBN 978-0-387-33334-2.
- ^ Safety Data Sheet: Xenon (PDF) (Report). Airgas. February 15, 2018.
- ^
LeBlanc, Adrian D.; Johnson, Philip C. (1971). "The handling of xenon-133 in clinical studies". Physics in Medicine and Biology. 16 (1): 105–9. S2CID 250787824.
- ^ a b Finkel, A. J.; Katz, J. J.; Miller, C. E. (April 1, 1968). "Metabolic and toxicological effects of water-soluble xenon compounds are studied". NASA. Retrieved 2022-03-18.
- .
- ^ Spangler, Steve (2007). "Anti-Helium – Sulfur Hexafluoride". Steve Spangler Science. Archived from the original on September 29, 2007. Retrieved 2007-10-04.
- ^
Yamaguchi, K.; Soejima, K.; Koda, E.; Sugiyama, N (2001). "Inhaling Gas With Different CT Densities Allows Detection of Abnormalities in the Lung Periphery of Patients With Smoking-Induced COPD". PMID 11742921.
- ^ Staff (August 1, 2007). "Cryogenic and Oxygen Deficiency Hazard Safety". Stanford Linear Accelerator Center. Archived from the original on June 9, 2007. Retrieved 2007-10-10.
External links
- Xenon at The Periodic Table of Videos(University of Nottingham)
- USGS Periodic Table – Xenon
- EnvironmentalChemistry.com – Xenon
- Sir William Ramsay's Nobel-Prize lecture (1904)

