Creutz–Taube complex
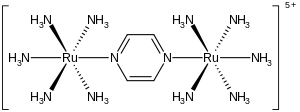
The Creutz–Taube ion is the
metal complex with the formula {[Ru(NH3)5]2(C4H4N2)}5+. This cationic species has been heavily studied in an effort to understand the intimate details of inner sphere electron transfer, that is, how electrons move from one metal complex to another. The ion is named after Carol Creutz, who first prepared the complex, and her thesis advisor Henry Taube, who received a Nobel Prize in Chemistry for this and related discoveries on electron transfer.[1][2]
Properties
The complex consists of two
intervalence charge-transfer band
.
Synthesis
The ion was originally isolated as the hydrated
tosylate salt [Ru(NH3)5]2(C4H4N2)(O3SC6H4CH3)5·3H2O. It is prepared in two steps via the Ru(III)-Ru(III) pyrazine complex:.[3]
- 2 [Ru(NH3)5Cl]2+ + C4H4N2 → {[Ru(NH3)5]2(C4H4N2)}6+ + 2 Cl−
- 2 {[Ru(NH3)5]2(C4H4N2)}6+ + Zn → 2 {Ru(NH3)5]2(C4H4N2)}5+ + Zn2+
The Creutz–Taube ion illustrates the advantages of ruthenium complexes for examining redox reactions. Ru(II) and Ru(III) ions can be interconverted at mild redox potentials. Both of these oxidation states are kinetically inert. Many analogues of this ion have been prepared using different bridging ligands.
References
- .
- ^ Taube, Henry (8 December 1983). "Electron Transfer between Metal Complexes". Nobel Lecture.
- ^ .
- PMID 11749392.
