Quaternium-15
| |
| Names | |
|---|---|
| IUPAC name
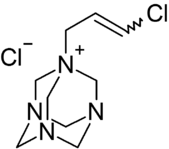
1-(3-Chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride
| |
Other names
| |
| Identifiers | |
| |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.102.448 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H16Cl2N4 | |
| Molar mass | 251.16 g·mol−1 |
| Hazards | |
| GHS labelling: | |
   
| |
| H228, H302, H315, H317, H361, H411[1] | |
| P210, P273, P280[1] | |
| Safety data sheet (SDS) | Sigma Aldrich[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Quaternium-15 (systematic name: hexamethylenetetramine chloroallyl chloride) is a
Both quaternium-15 and formaldehyde release agents have been the subjects of controversy. They are often banned in US and Europe.[2][3]
It can be found under a variety of names, including Dow Chemical Company: Dowicil 200 (cis isomer only), Dowicil 75 and Dowicil 100 (both a mix of cis and trans isomers).
Synthesis
Quaternium-15 can be prepared by treating
Applications
The isolated cis-compound is used primarily in cosmetic applications, with a maximum permitted concentration in the EU of 0.2%. The mixed product (cis- and trans-) is used in a wider range of formulations such as: emulsifiable metal-cutting fluids, latex and emulsion paints, liquid floor polishes and floor waxes, and glues and adhesives.
Safety concerns
Quaternium-15 has been banned in the EU since 2017 and a bill was introduced in the US in 2017 to require the FDA to investigate its safety.[4][5]
Allergic reaction
Quaternium-15 is an
Although quaternium-15 releases low amounts of formaldehyde.[10] Even so, Johnson & Johnson announced plans to phase out its use of quaternium-15 in cosmetic products by 2015 in response to consumer pressure.[11][12]
See also
References
- ^ a b c Sigma-Aldrich Co., 1-(cis-3-Chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride. Retrieved on 2014-10-07.
- S2CID 39758546.
- S2CID 28278068.
- ^ "European Commission notifies bans, restrictions on CMRS in cosmetics". Retrieved 2 December 2019.
- ^ "The cosmetics industry has avoided strict regulation for over a century. Now rising health concerns has FDA inquiring". Retrieved 2 December 2019.
- PMID 16197434
- ^ New Zealand Dermatological Society. "Quaternium-15 contact allergy". DermNet NZ. Retrieved 2007-05-31.
- ^ E. Warshaw, et al. "Contact dermatitis of the hands: Cross-sectional analyses of North American Contact Dermatitis Group Data, 1994–2004". Journal of the American Academy of Dermatology, Volume 57, Issue 2, pp. 301–314
- S2CID 24088485.
- ^ "Formaldehyde". American Cancer Society. Retrieved 3 March 2016.
- ^ "Johnson & Johnson to phase out potentially harmful chemicals by 2015". CBS News. Retrieved 3 March 2016.
- ^ Thomas, Katie (17 January 2014). "The 'No More Tears' Shampoo, Now With No Formaldehyde". The New York Times. Retrieved 3 March 2016.
