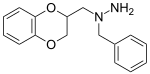
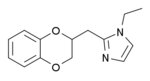
Benzodioxan
Appearance

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydro-1,4-benzodioxine | |
| Other names
Dihydrobenzodioxin; 1,4-Benzodioxane; Benzo-1,4-dioxane; Ethylene o-phenylene dioxide; Pyrocatechol ethylene ether
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The benzodioxans are a group of
Derivatives
Some derivatives of 1,4-benzodioxan are used as pharmaceuticals including:[4][5][6][7]
See also
References
- National Library of Medicine. Archivedfrom the original on 2022-09-05. Retrieved 2022-09-05.
- ^ "TECHNICAL FACT SHEET – 1,4-DIOXANE" (PDF). Technical Fact Sheet. 51 (6): 9. 2017. Archived (PDF) from the original on 2022-08-13. Retrieved 2022-09-05 – via United States Environmental Protection Agency.
- United States Department of Health.
- PMID 21782456.
- ^ "1,4-benzodioxan derivatives their preparation and pharmaceutical compositions containing them - Patent IL-43272-A - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-09-05.
- PMID 27658794.
- PMID 18683910.
External links
 Media related to Benzodioxan at Wikimedia Commons
Media related to Benzodioxan at Wikimedia Commons