Source: Wikipedia, the free encyclopedia.
Etamycin
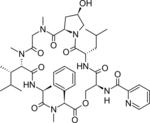
|
| Names
|
| Other names
Viridogrisein I, Etamycin A, Neoviridogrisein IV, Antibiotic K-179, Antibiotic F-1370A
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
|
|
|
| UNII
|
|
InChI=1S/C44H62N8O11/c1-23(2)19-30-42(60)52-21-29(53)20-31(52)43(61)49(8)22-33(55)50(9)36(25(5)24(3)4)40(58)46-26(6)41(59)51(10)37(28-15-12-11-13-16-28)44(62)63-27(7)34(38(56)47-30)48-39(57)35-32(54)17-14-18-45-35/h11-18,23-27,29-31,34,36-37,53-54H,19-22H2,1-10H3,(H,46,58)(H,47,56)(H,48,57)/t25?,26-,27-,29+,30+,31+,34-,36-,37-/m0/s1 Key: SATIISJKSAELDC-QPFUEGANSA-N InChI=1/C44H62N8O11/c1-23(2)19-30-42(60)52-21-29(53)20-31(52)43(61)49(8)22-33(55)50(9)36(25(5)24(3)4)40(58)46-26(6)41(59)51(10)37(28-15-12-11-13-16-28)44(62)63-27(7)34(38(56)47-30)48-39(57)35-32(54)17-14-18-45-35/h11-18,23-27,29-31,34,36-37,53-54H,19-22H2,1-10H3,(H,46,58)(H,47,56)(H,48,57)/t25?,26-,27-,29+,30+,31+,34-,36-,37-/m0/s1 Key: SATIISJKSAELDC-QPFUEGANBR
|
|
|
| Properties
|
|
|
C44H62N8O11
|
| Molar mass
|
879.025 g·mol−1
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Chemical compound
Etamycin, also known as viridogrisein,actinomycete.
[2] Etamycin was first isolated from a
Streptomyces species in 1957 by Lawson and co-workers.
[3]
Notes

