Heavy-chain antibody
A heavy-chain antibody is an antibody which consists only of two heavy chains and lacks the two light chains usually found in antibodies.
In common antibodies, the
Discovery
In 1989 a group of biologists led by
In cartilaginous fishes
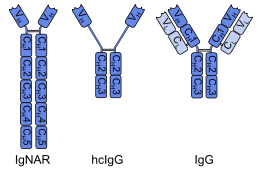
The immunoglobulin new antigen receptor (IgNAR) of
Sharks, and possibly other cartilaginous fishes, have immunoglobulin M (IgM) and immunoglobulin W (IgW) as well, both types with two heavy and two light chains.[5]
In camelids
The only mammals with heavy-chain (
About 50% of the antibodies in camelids are of the ordinary mammalian heavy/light-chain type.[7] It is not known whether any type of animal has only heavy-chain antibodies and completely lacks the common type with two heavy and two light chains.
Heavy-chain camelid antibodies have been found to be just as specific as a regular antibody and in some cases they are more robust. As well, they are easily isolated using the same phage panning procedure used for traditional antibodies, allowing them to be cultured
References
- ^ PMID 17704915.
- ^ S2CID 4265902.
- S2CID 4304231.
- ^ S2CID 25137728.
- PMID 19554288.
- PMID 12543123.
- ^ "Nanobodies". Nanobody.org. 2006.
- ^ Ghannam, A., Kumari, S., Muyldermans, S., & Abbady, A. Q. (2015). Camelid nanobodies with high affinity for broad bean mottle virus: a possible promising tool to immunomodulate plant resistance against viruses. Plant Molecular Biology, 1-15.
External links
- Wikilite: Biology of immunoglobulin light chains
