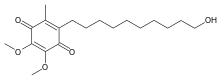
Idebenone
 | |
| Clinical data | |
|---|---|
| Trade names | Catena, Raxone, Sovrima |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <1% (high first pass effect) |
| Protein binding | >99% |
| Elimination half-life | 18 hours |
| Excretion | Urine (80%) and feces |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Idebenone (pronounced eye-deb-eh-known, trade names Catena, Raxone, Sovrima, among others) is a
Chemically, idebenone is an
Uses
Indications that are or were approved in some territories
Nootropic effects and Alzheimer's disease
Idebenone improved learning and memory in experiments with mice. but larger studies with hard endpoints are missing.
Research on idebenone as a potential therapy of Alzheimer's disease have been inconsistent, but there may be a trend for a slight benefit.[7][8] In May 1998, the approval for this indication was cancelled in Japan due to the lack of proven effects. In some European countries, the drug is available for the treatment of individual patients in special cases.[1]
Friedreich's ataxia (Sovrima)
Preliminary testing has been done in humans and found idebenone to be a safe treatment for Friedreich's ataxia (FA), exhibiting a positive effect on
The drug was approved for FA in Canada in 2008 under conditions including proof of efficacy in further clinical trials.[12] However, on February 27, 2013, Health Canada announced that idebenone would be voluntarily recalled as of April 30, 2013 by its Canadian manufacturer, Santhera Pharmaceuticals, due to the failure of the drug to show efficacy in the further clinical trials that were conducted.[13] In 2008, the European Medicines Agency (EMA) refused a marketing authorisation for this indication.[1] As of 2013 the drug was not approved for FA in Europe[14] nor in the US, where, as of February 2023, there is only one approved treatment.[15]
Leber's hereditary optic neuropathy (Raxone)
Leber's hereditary optic neuropathy (LHON) is a mitochondrially inherited (mother to all offspring) degeneration of retinal ganglion cells (RGCs) and their axons that leads to an acute or subacute loss of central vision; this affects predominantly young adult males. Santhera completed a Phase III clinical trial in this indication in Europe with positive results,[16] and submitted an application to market the drug to European regulators in July 2011.[17] It is approved by EMA for this indication and was designated an orphan drug in 2007.[4]
Indications being explored
Duchenne muscular dystrophy (Catena)
After experiments in mice[18] and preliminary studies in humans, idebenone has entered Phase II clinical trials in 2005[3] and Phase III trials in 2009.[19]
Other neuromuscular diseases
Phase I and II clinical trial for the treatment of
As of 2022, a phase III clinical trial is ongoing for the treatment of Parkinson's disease.[23]
Life style
Idebenone is claimed to have properties similar to CoQ10 in its
Pharmacology
In cellular and tissue models, idebenone acts as a transporter in the
Pharmacokinetics
Idebenone is well absorbed from the gut but undergoes excessive
References
- ^ a b c d e CHMP Assessment Report for Sovrima (PDF) (Report). European Medicines Agency. 20 November 2008. pp. 6, 9–11, 67f.
- ^ Clinical trial number NCT00229632 for "Idebenone to Treat Friedreich's Ataxia" at ClinicalTrials.gov
- ^ a b Clinical trial number NCT00654784 for "Efficacy and Tolerability of Idebenone in Boys With Cardiac Dysfunction Associated With Duchenne Muscular Dystrophy (DELPHI)" at ClinicalTrials.gov
- ^ a b "Raxone". www.ema.europa.eu. 17 September 2018. Retrieved 12 July 2019.
- PMID 11270979.
- PMID 9706371.
- PMID 11819153.
- S2CID 46987059.
- S2CID 24749816.
- S2CID 73285881.
- S2CID 36556782.
- ^ "Heath Canada Fact Sheet - Catena". Archived from the original on 19 June 2014.
- ^ Voluntary Withdrawal of Catena from the Canadian Market
- ^ Margaret Wahl for Quest Magazine, MAY 28, 2010. FA Research: Idebenone Strikes Out Again
- ^ FDA approves first treatment for Friedreich’s ataxia
- PMID 21788663.
- ^ Staff (26 July 2011). "Santhera publishes pivotal trial results of idebenone and goes for EU approval". European Biotechnology News. Archived from the original on 2013-02-17.
- PMID 18784063.
- ^ Clinical trial number NCT01027884 for "Phase III Study of Idebenone in Duchenne Muscular Dystrophy (DMD) (DELOS)" at ClinicalTrials.gov
- ^ Clinical trial number NCT00887562 for "Study of Idebenone in the Treatment of Mitochondrial Encephalopathy Lactic Acidosis & Stroke-like Episodes (MELAS)" at ClinicalTrials.gov
- ^ Clinical trial number NCT00950248 for "Double Blind Placebo-Controlled Phase I/II Clinical Trial of Idebenone in Patients With Primary Progressive Multiple Sclerosis (IPPoMS)" at ClinicalTrials.gov
- PMID 32784117.
- PMID 35527571.
- S2CID 2394666.
- PMID 3410376.
