Cordycepin
| |
| Names | |
|---|---|
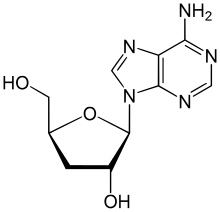
| IUPAC name
3′-Deoxyadenosine
| |
| Systematic IUPAC name
(2S,3R,5S)-2-(6-Amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolan-3-ol | |
| Other names
Cordycepine
9-(3-Deoxy-β-D-ribofuranosyl)adenine 3-dA | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.000.720 |
IUPHAR/BPS |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H13N5O3 | |
| Molar mass | 251.246 g·mol−1 |
| Melting point | 225.5 °C (437.9 °F; 498.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cordycepin, or 3'-deoxyadenosine, is a
Cordycepin is produced in cordyceps as a means of infecting insect populations, due to cordycepin's biological activity[4]
Because cordycepin is similar to adenosine, some enzymes cannot discriminate between the two.[citation needed] It can therefore participate in certain biochemical reactions (for example, 3-dA can trigger the premature termination of mRNA synthesis).[5][6] By acting as an adenosine analog, cordycepin was found to be the most potent molecular circadian clock resetter out of several screened compounds.[7]
Cordycepin has displayed cytotoxicity against some leukemic cell lines in vitro.[8][9][10] Additionally, cordycepin has been shown to display an effect in some types of other cancers, such as lung,[11] renal,[12] colon,[13] and breast cancer.[14] Cordycepin has been shown to reduce viable A549 lung cancer cell populations by 50%.[11]
Cordycepin has been found to produce rapid, robust imipramine-like antidepressant effects in animal models of depression, and these effects, similarly to those of imipramine, are dependent on enhancement of AMPA receptor signaling.[15]
Cordycepin has been shown to have anti-inflammatory qualities,[16] as well as the ability to defend against injury from cerebral ischemia in mice.[17]
See also
References
- PMID 14796634.
- S2CID 90915876.
- PMID 19051361.
- PMID 31890138.
- PMID 5783871.
- PMID 23118416.
- S2CID 218533423.
- ^ National Cancer Institute (2011-02-02). "Definition of cordycepin". NCI Drug Dictionary. Retrieved 21 December 2015.
- PMID 10609556.
- PMID 25442260.
- ^ .
- PMID 29045468.
- PMID 23941773.
- PMID 31454995.
- PMID 26443809.
- S2CID 224828245.
- PMID 21554870.
