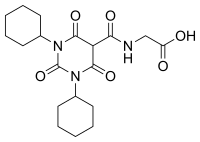
Daprodustat
 | |
| Clinical data | |
|---|---|
| Trade names | Duvroq, Jesduvroq |
| Other names | GSK1278863 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a623010 |
| License data |
|
Hypoxia-inducible factor prolyl hydroxylase inhibitor | |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
JSmol) | |
| |
| |
Daprodustat, sold under the brand name Duvroq among others, is a
The most common side effects include
Daprodustat was approved for medical use in Japan in June 2020,[5][6] and in the United States in February 2023.[2][3][7][8] making it the first oral treatment for anemia caused by chronic kidney disease for adults in the US.[3] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[9]
Medical uses
Daprodustat is
Daprodustat increases erythropoietin levels.[3]
Adverse effects
The FDA label for daprodustat has a boxed warning for an increased risk of thrombotic vascular (blood clotting) events including death, heart attack, stroke, and blood clots in the lung, legs, or dialysis access site.[4]
The most common side effects include high blood pressure, thrombotic vascular events, abdominal pain, dizziness, and allergic reactions.[3][4]
History
The efficacy and safety of daprodustat were evaluated in 2,964 adults with anemia due to chronic kidney disease on dialysis and receiving an erythropoiesis-stimulating agent at the time of study entry in a randomized, sponsor-blind, active-controlled, global, multicenter, event-driven clinical trial (ASCEND-D; NCT02879305).
The FDA granted the approval of Jesduvroq to GlaxoSmithKline LLC.[3]
Society and culture
Due to its potential applications in
Research
Daprodustat is in phase III clinical trials for the treatment of anemia caused by chronic kidney disease.[12][13][14]
References
- FDA. Retrieved 22 October 2023.
- ^ a b c d e f "Jesduvroq- daprodustat tablet, film coated". DailyMed. 1 February 2023. Archived from the original on 11 February 2023. Retrieved 11 February 2023.
- ^ a b c d e f g h i "FDA Approves First Oral Treatment for Anemia Caused by Chronic Kidney Disease for Adults on Dialysis". U.S. Food and Drug Administration (FDA) (Press release). 1 February 2023. Archived from the original on 4 February 2023. Retrieved 3 February 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f g h i j "Drug Trials Snapshots: Jesduvroq". U.S. Food and Drug Administration (FDA). 1 February 2023. Archived from the original on 24 February 2024. Retrieved 24 February 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- PMID 32880805.
- ^ "GSK receives first regulatory approval for Duvroq (daprodustat) in Japan for patients with anaemia due to chronic kidney disease" (Press release). GSK. 29 June 2020. Archived from the original on 4 February 2023. Retrieved 29 March 2021.
- PMID 36790833.
- ^ "Jesduvroq (daprodustat) approved by US FDA for anemia of chronic kidney disease in adults on dialysis" (Press release). GSK US. 1 February 2023. Archived from the original on 4 February 2023. Retrieved 24 February 2024.
- ^ New Drug Therapy Approvals 2023 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2024. Archived from the original on 10 January 2024. Retrieved 9 January 2024.
- ^ Clinical trial number NCT02879305 for "Anemia Studies in Chronic Kidney Disease: Erythropoiesis Via a Novel Prolyl Hydroxylase Inhibitor Daprodustat-Dialysis (ASCEND-D)" at ClinicalTrials.gov
- PMID 26361079.
- S2CID 32493057.
- PMID 28928122.
- S2CID 243761990.
