Ocimene
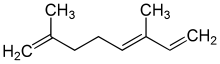 α-Ocimene
| |
 cis-β-Ocimene
| |
 trans-β-Ocimene
| |
| Names | |
|---|---|
| IUPAC names
α: (3E)-3,7-Dimethylocta-1,3,7-triene
cis-β: (3Z)-3,7-Dimethylocta-1,3,6-triene trans-β: (3E)-3,7-Dimethylocta-1,3,6-triene | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider |
|
ECHA InfoCard
|
100.034.205 |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C10H16[1] | |
| Molar mass | 136.24 g/mol |
| Density | 0.800 g/cm3 |
| Melting point | 50 °C (122 °F; 323 K) |
| Boiling point | mix of isomers: 100 °C at 70 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ocimenes are a group of
stereoisomeric forms, cis and trans, with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent, and are believed to act as plant defense and have anti-fungal properties.[2] Like the related acyclic terpene myrcene, ocimenes are unstable in air.[3]
Like other terpenes, the ocimenes are nearly insoluble in water, but soluble in common organic solvents.
The
, ὤκιμον (ṓkimon).References
- ^ "CID 5281553 -- PubChem Compound Summary". Retrieved 2008-02-17.
- ^ SCLabs, Beyond Aroma: Terpenes in cannabis Archived 2016-06-15 at the Wayback Machine
- ISBN 978-3-527-31786-8. Retrieved 2 August 2013.
Acyclic monoterpenoid trienes such as p-myrcene and configurational isomers of p-ocimene are found in the oils of basil (leaves of Ocimum basilicum, Labiatae), bay (leaves of Fimenta acris, Myrtaceae), hops (strobiles of Humulus lupulus, ...
