Organosulfate

In
Applications
Alkyl sulfates are commonly used as an anionic surfactant in liquid soaps and detergents used to clean wool, as surface cleaners, and as active ingredients in laundry detergents, shampoos and conditioners. They can also be found in household products such as toothpaste, antacids, cosmetics and foods. Generally they are found in consumer products at concentrations ranging from 3-20%. In 2003 approximately 118,000 t/a of alkyl sulfates were used in the US[1]
Synthetic organosulfates
A common example is
Alkylsulfate can be produced from alcohols, which in turn are obtained by
- ClSO3H + ROH → ROSO3H + HCl
Alternatively, alcohols can be converted to the half sulfate esters using sulfur trioxide:[4]
- SO3 + ROH → ROSO3H
Some organosulfates can be prepared by the Elbs persulfate oxidation of phenols and the Boyland–Sims oxidation of anilines.
Dialkylsulfates

A less common family of organosulfates have the formula RO-SO2-OR'. They are prepared from sulfuric acid and the alcohol. The main examples are
Natural sulfate esters
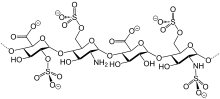
Several classes of sulfate esters exist in nature. Especially common are sugar derivatives such as keratan sulfate, chondroitin sulfate, and the anticoagulant heparin.[6] Post-translational modifications of some proteins entail sulfation, often at the phenol group of tyrosine residues.[7] A steroidal sulfate is estradiol sulfate, a latent precursor to the hormone estrogen.
A major portion of soil sulfur is in the form of sulfate esters.[8]
Metabolism
Sulfate is an inert anion, so nature activates it by the formation of ester derivative of adenosine 5'-phosphosulfate (APS) and
Safety
Because they are widely used in commercial products, the safety aspects of organosulfates are heavily investigated.[10]
Human Health
Alkyl sulfates if ingested are well-absorbed and are metabolized into a C3, C4 or C5 sulfate and an additional metabolite. The highest irritant of the alkyl sulfates is sodium laurylsulfate, with the threshold before irritation at a concentration of 20%.
Laboratory studies have not found alkyl sulfates to be
Environment
The primary disposal of alkyl sulfate from used commercial products is wastewater. The concentration of alkylsulfates in effluent from waste water treatment plants (WWTP) has been measured at 10 micrograms per litre (5.8×10−9 oz/cu in) and lower. Alkyl sulfates biodegrade easily, even starting likely before reaching the WWTP. Once at the treatment plant, they are rapidly removed by
In terms of thermal stability, alkyl sulfates degrade well before reaching their boiling point due to low vapor pressure (for C8-18 from 10-11 to 10-15 hPa). Soil sorption is proportional to carbon chain length, with a length of 14 and more having the highest sorption rate. Soil concentrations have been found to vary from 0.0035 to 0.21 milligrams per kilogram (5.6×10−8 to 3.4×10−6 oz/lb) dw.
References
- ^ CEH (October 2004). "Surfactants, household detergents and their raw materials". CEH Marketing Research Report.
- .
- ISBN 978-3527306732.
- ^ PMID 16895327.
- PMID 791238.
- PMID 12730193.
- .
- ISBN 0-13-520875-0.
- ^ a b SDA/Alkylsulfate Consortium (2007). "SIDS Initial Assessment Profile. SIAM 25: Alkyl Sulfates, Alkane Sulfonates, and α-Olefin sulfonates" (PDF). OECD SIDS. Helsinki. Archived from the original (PDF) on 2016-03-03. Retrieved 2011-10-14.
- ^ DE/ICCA (2009). "SIDS Initial Assessment Profile SIAM 25: Alkyl Sulfates, Alkane Sulfonates, and α-Olefin sulfonates". OECD.
- PMID 21463896.
