Phosphonium


4, the parent phosphonium cation.
In
Types of phosphonium cations
Protonated phosphines
The parent phosphonium is PH+
4 as found in the iodide salt, phosphonium iodide. Salts of the parent PH+
4 are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride:
- PH3 + HCl + 4 CH2O → P(CH
2OH)+
4Cl−
Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines:
- PR3 + H+ → HPR+
3
The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl.[2]
Tetraorganophosphonium cations
The most common phosphonium compounds have four organic substituents attached to phosphorus. The quaternary phosphonium cations include tetraphenylphosphonium, (C6H5)4P+ and tetramethylphosphonium P(CH
3)+
4.


Quaternary phosphonium cations (PR+
4) are produced by alkylation of organophosphines.
- PPh3 + CH3Br → [CH3PPh3]+Br−
The methyl group in such phosphonium salts is mildly acidic, with a pKa estimated to be near 15:[5]
- [CH3PPh3]+ + base → CH2=PPh3 + [Hbase]+
This deprotonation reaction gives Wittig reagents.[6]
Solid
4PCl−
6, that is, a salt containing the tetrachlorophosphonium cation.[7][8]
- PCl5 ⇌ PCl+
4 + Cl−
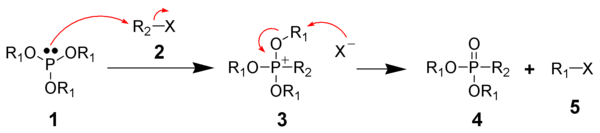
Alkoxyphosphonium salts: Arbuzov reaction
The
Uses
Textile finishes

Phase-transfer catalysts and precipitating agents
Organic phosphonium cations are lipophilic and can be useful in
4) are soluble in polar organic solvents. One example is the perrhenate (PPh4[ReO4]).[16]
Reagents for organic synthesis
Wittig reagents are used in organic synthesis. They are derived from phosphonium salts. A strong base such as butyllithium or sodium amide is required for the deprotonation:
- [Ph3P+CH2R]X− + C4H9Li → Ph3P=CHR + LiX + C4H10
One of the simplest ylides is methylenetriphenylphosphorane (Ph3P=CH2).[6]
The compounds Ph3PX2 (X = Cl, Br) are used in the Kirsanov reaction.[17] The Kinnear–Perren reaction is used to prepare alkylphosphonyl dichlorides (RP(O)Cl2) and esters (RP(O)(OR′)2). A key intermediate are alkyltrichlorophosphonium salts, obtained by the alkylation of phosphorus trichloride:[18]
- RCl + PCl3 + AlCl3 → [RPCl3]+AlCl−
4
Ammonia production for "green hydrogen"
The main industrial procedure for the production of ammonia today is the thermal Haber-Bosch process, which generally uses fossil gas as a source of hydrogen, which is then combined with nitrogen to produce ammonia. In 2021, Professor Doug MacFarlane and collaborators Alexandr Simonov and Bryan Suryanto of Monash University devised a method of producing green ammonia that has the potential to make Haber-Bosch plants obsolete.[19] Their process is similar to the electrolysis approach for producing hydrogen. While working with local company Verdant, which wanted to make bleach from saltwater by electrolysis, Suryanto discovered that a tetraalkyl phosphonium salt allowed the efficient production of ammonia at room temperature.[20]
See also
- Ammonium (NH+
4) - Arsonium (AsH+
4) - Hydronium (H3O+)
- Onium compounds
- Organophosphorus chemistry
References
- ISBN 978-0-444-89307-9.
- PMID 17245785.
- ^ ISBN 9780470132494.
- .
- .
- ^ .. Describes Ph3P=CH2.
- ISBN 978-0-12-352651-9.
- .
- .
- PMID 25384344.
- .
- S2CID 98355305.
- ^ "Frequently asked questions: What is the PROBAN® process?". Rhodia Proban. Archived from the original on December 7, 2012. Retrieved February 25, 2013.
- .
- ISBN 9780470132623.
- ISBN 978-3527306732.
- ^ Breakthrough brings green ammonia production closer to reality
- ^ Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle
