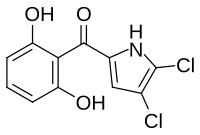
Pyoluteorin
Pyoluteorin is a natural
Biosynthesis
Pyoluteorin is synthesized from an NRPS/PKS hybrid pathway. The resorcinol ring is derived from a type I PKS[6][7] while the dichloropyrrole[clarification needed] moiety is derived from a type II NRPS.[8] Pyoluteorin biosynthesis begins with the activation of L-proline to prolyl-AMP by the adenylation domain PltF. With prolyl-AMP still in the active site, the active form of the peptidyl carrier protein PltL binds to PltF. Then PltF catalyzes the aminoacylation of PltL by attaching L-proline to the thiol of the 4’phosphopantetheine arm of PltL.[9] Next, the dehydrogenase PltE desaturates the prolyl moiety on PltL to create pyrrolyl-PltL. The halogenation domain PltA then dichlorinates the pyrrole moiety first at position 5 and then at position 4 in a FADH2 dependent manner.[10] The dichloropyrroyl residue is then transferred to the type I PKS PltB and PltC, however, the mechanism of transfer is unknown. The addition of 3 malonyl-CoA monomers, cyclization, and release by the thioesterase PltG gives pyoluteorin.