Strecker amino acid synthesis
| Strecker synthesis | |
|---|---|
| Named after | Adolph Strecker |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | strecker-synthesis |
| RSC ontology ID | RXNO:0000207 |
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with cyanide in the presence of ammonia. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid.[1][2] The method is used for the commercial production of racemic methionine from methional.[3]

Primary and secondary amines also give N-substituted amino acids. Likewise, the usage of ketones, instead of aldehydes, gives α,α-disubstituted amino acids.[4]
Reaction mechanism
In the first part of the reaction, the
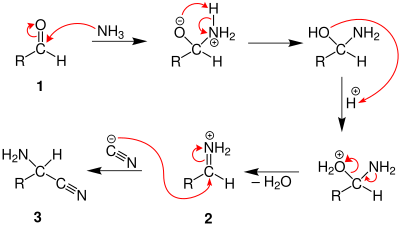
In the second part of the Strecker synthesis, the nitrile nitrogen of the aminonitrile is protonated, and the nitrile carbon is attacked by a water molecule. A 1,2-diamino-diol is then formed after proton exchange and a nucleophilic attack of water to the former nitrile carbon. Ammonia is subsequently eliminated after the protonation of the amino group, and finally the deprotonation of a hydroxyl group produces an amino acid.

Asymmetric Strecker reactions
One example of the Strecker synthesis is a multikilogram scale synthesis of an
- (CH3)2CHC(O)CH3 + HCN + NH3 → (CH3)2CHC(CN)(NH2)CH3 + H2O
The initial reaction product of 3-methyl-2butanone with

Catalytic asymmetric Strecker reaction can be effected using thiourea-derived
History
The German chemist Adolph Strecker discovered the series of chemical reactions that produce an amino acid from an aldehyde or ketone.[9][10] Using ammonia or ammonium salts in this reaction gives unsubstituted amino acids. In the original Strecker reaction acetaldehyde, ammonia, and hydrogen cyanide combined to form after hydrolysis alanine. Using primary and secondary amines in place of ammonium was shown to yield N-substituted amino acids.[10]
The classical Strecker synthesis gives racemic mixtures of α-amino acids as products, but several alternative procedures using asymmetric auxiliaries[11] or asymmetric catalysts[12][13] have been developed.
The asymmetric Strecker reaction was reported by Harada in 1963.
Commercial syntheses of amino acids
Several methods exist to synthesize amino acids aside from the Strecker synthesis.[17][3]
The commercial production of amino acids, however, usually relies on mutant bacteria that overproduce individual amino acids using glucose as a carbon source. Otherwise amino acids are produced by enzymatic conversions of synthetic intermediates.
