Talk:Ferrocene
chemicals. To participate, help improve this article or visit the project page for details on the project. | ||
| High | This article has been rated as High-importance on the project's importance scale. | |
| This is a core article in the WikiProject Chemicals worklist. | ||
lithiation
the reference for the monolithiation of ferrocene is refuted by the author in a later paper where that result could not be reproduced. See Denis Guillaneux, Henri B. Kagan, Journal of Organic Chemistry 1995, vol. 60, No. 8, pp. 2502-2505; also included in that reference is a better description of the lithiation of ferrocene.
- ]
Photographs
the photograph of the powdered ferrocene does little more than show the color of the substance. I believe an image of the crystalline substance would be more useful. For example,

Jrb737 (talk) 08:12, 1 December 2015 (UTC)
Diagrams
I've created and uploaded two new images of ferrocene, a 2D black and white structure and a 3D colour structure. I can modify them if required. I've drawn them in an orientation which allows them to be compared side-by-side.
I think the current line drawing of ferrocene is a bit dodgy, because it makes the C-C bonds look the same as the C-Fe bonds. Having looked in my main chemistry textbooks (Organic Chemistry, Clayden, Greeves, Warren and Wothers and Chemistry of the Elements, Greenwood & Earnshaw), it seems they prefer the shorthand notation of drawing a single bond from the centre of each Cp ring.
I don't want to remove the image from the article, because I imagine many people like it and find it useful. But if you are like me and would prefer a slightly different diagram, comment here. I've created a new image which is how I think the line drawing would look if it were more accurate. I don't like it though, because it's messy! What do you all think?
Ben 22:52, 26 February 2006 (UTC)
Sorry. I don't know how to use wikipedia. But the lowest energy state of ferrocene is not the staggered conformation, it is the eclipsed conformation that is the lowest energy state. So, all of the drawings on this page are not the ground state. Usually molecules are drawn in the ground state unless they have been somehow excited. You should be able to confirm this in Shriver and Atkins Inorganic chemistry. Thanks.
Pauson v. Paulson
I have a copy of the Nature 1951, December 15, page 1309 in front of me. The authors names are T. J. Kealy and P. L. Pauson. NOT Paulson. Now, having said that subsequent papers are under the name Paulson, such as the retrospective report in Journal of Organometallic Chemistry. In that report he does not cite any literature, so we cannot see how he cited his Nature paper. He was a refugee and possibly was still deciding on how to transliterate his name. ChemAbs lists his name as PauLson on this paper, despite what I can see with my own eyes. Beats me.--Smokefoot 17:00, 1 June 2006 (UTC)
- take back some of what I said - he always published under the name Pauson. CAS just messed up. The Duquesne Univ website use the L-free spelling of Pauson.--Smokefoot 17:41, 1 June 2006 (UTC)
Sublimation
Does anybody know what
Ferrocene, being readily sublimed, can be used to deposit certain kinds of fullerenes
means?? --Chris 21:53, 12 June 2006 (UTC)
- No clue here .. what about remove? Maybe a next input will be clearer, does not make sense now? --Dirk Beetstra T C 22:24, 12 June 2006 (UTC)
- I would suggest that the fullerenes can be incorporated into a solid sample of ferrocene (eg present during a recrystallisation of ferrocene). When the ferrocene/fullerene sample is heated, ferrocene sublimes leaving the fullerene behind.--Artorius 10:48, 14 June 2006 (UTC)
- Ferrocene is used, so I am told, to deposit multiwalled nanotubes. We probably remove this bit as it is peripheral, but apparently Fc gas decomposes to give the Fe that catalyzes the decomposition of toluene or other carbon donors in a flowing hot tube. The factoid is not particularly critical to the article, and possibly better for a buckytube article. --Smokefoot 11:17, 14 June 2006 (UTC)
Edits of March, 2008
The following image was removed/replaced in my editing. The content is highly specialized, but nonetheless these are factoids so I didnt want to unilaterally remove them from the main article.
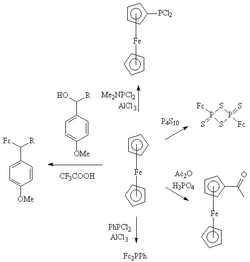
GA review
I've signed up to review this. It may be a day or two before I get round to commenting in detail, so there's time for a final careful copy edit (:
Also the intro strikes me as not really fulfilling
]- and the big reaction schemes are substandard and do not reflect main-stream views of the important (i.e. widely used) rxns. I am going to insert new ones.--Smokefoot (talk) 17:22, 27 March 2008 (UTC)
Good Article nomination
- herefor criteria)
- It is reasonably well written.
- a (prose):
 b (MoS):
b (MoS): 
- a (prose):
- It is factually accurate and verifiable.
- a (references):
 b (citations to reliable sources):):
b (citations to reliable sources):): c (OR
c (OR
- a (references):
- It is broad in its coverage.
- a (major aspects):
 b (focused):
b (focused): 
- a (major aspects):
- It follows the neutral point of viewpolicy.
- Fair representation without bias:

- Fair representation without bias:
- It is stable.
- No edit wars etc.:

- No edit wars etc.:
- It is illustrated by images, where possible and appropriate.
- a (images are tagged and non-free images have suitable captions):

- a (images are tagged and non-free images have
- Overall:
- Pass/Fail:

- Pass/Fail:
Magnetic Properties
I feel that addition of something about the magnetic properties of this compound, and perhaps its analogues, would benefit the article. Checking Google, there seems to have been a lot of papers published on this, but I didn't quickly find any simple summary by an expert in the field on its macroscopic magnetic properties as a regular wiki-reading Joe might be curious. For example, is there a relatively non-toxic (very stable in air, next to no vapor pressure, no contact toxicity, etc - basically OK as long as you don't chug the stuff, ya know?) ferrocene that can be dissolved into something like paraffin to make a cool-looking ferrofluid for visual effect fun with magnets? Zaphraud (talk) 05:24, 13 March 2009 (UTC)
- Problem is, Ferrocene isn't magnetic (at least, not paramagnetic). And its derivatives don't really fulfil your list of criteria. Sorry! Chris (talk) 08:57, 13 March 2009 (UTC)
Staggered vs. Eclipsed conformation
Although x-ray crystallography data (in the monoclinic space group) points to the Cp rings being in a staggered conformation, it has been shown through gas phase electron diffraction 1,2 and computational studies 3 that the gas phase equilibrium state of ferrocene is actually with the Cp rings in the eclipsed conformation. The staggered conformation is believed to be most stable in the condensed phase due to crystal packing.
There is already a small blurb about the Cp rings being able to freely rotate, but there is no mention of which conformation is most stable in which phase or ferrocene's small barrier of rotation. Just thought that this could be an area that is expanded upon. — Preceding unsigned comment added by 129.74.115.92 (talk) 15:08, 31 May 2012 (UTC)
The eclipsed conformation is electronically preferred as it has better orbital overlap, whereas the staggered conformation is sterically preferred and is better for Cp with larger substituents. — Preceding unsigned comment added by 86.1.161.90 (talk) 21:39, 9 June 2020 (UTC)
- Feel free to add this to the article yourself. You seem knowledgeable. If you would like any help formatting the references or wish to discuss the proposed addition in more detail, many editors (including me) would be happy to help. Secondary sources, i.e. review articles and especially textbooks are the best references - see if you can find anything of that nature on the topic. Thanks for your excellent suggestions. --Ben (talk) 18:47, 31 May 2012 (UTC)
Ferrocenium versus Ferricinium
These terms, rather confusingly, both denote the SAME +1 cation. Ferricinium was used in the earlier literature and British sources such as Britannica, but a Google search gives far more uses of Ferrocenium today. I put in a brief explanation in the Ferrocene article, but the Ferrocenium article (which is rather vestigial, being mainly about ferrocenium tetrafluoroborate) and the Ferrocenium entry in Wiktionary also need to be fixed. Does anyone know if this is an official change in standard nomenclature, like carbonium being changed to carbocation? As an undergraduate research assistant I synthesized ferrocene and ferricinium compounds during the 1960s, when the ion was always called ferricinium.CharlesHBennett (talk) 12:28, 6 November 2014 (UTC)CharlesHBennett (talk) 13:24, 6 November 2014 (UTC)
- Useful comments. I have often wondered. Some small issues with your edit:
"originally called ferricinium, but now more commonly ferrocenium (these terms denote the same ion, contrary to what one would expect from the fact that ferric and ferrous denote different ions of a single iron atom)."
- "Now" is not a useful term in an encyclopedia. Similarly "recent" etc
- your comments "contrary to what one would expect from the fact that ferric and ferrous denote different ions of a single iron atom) are conjecture." They seem logical, but probably doesnt belong in Wikipedia.
Now about ferrocenium being "vestigial". Horrors. It is a rather specialized topic and the BF4 salt is the main one. So rather than having readers consult a generic and thin article on the cation, we (I) shunt them to the salt they probably want. --Smokefoot (talk) 17:37, 6 November 2014 (UTC)
Original synthesis and reaction equation in article
The reaction equation shows Fe(II)Cl2 as a reagent, but the original synthesis used ferric chloride (Fe(III)Cl3). Should be adapted to each other. --ZdBdLaLaLa (talk) 17:07, 10 September 2015 (UTC)
- The Pauson and Keely synthesis used iron(II) chloride, not the iron(III) salt. I have corrected the text and equation, and I see from the history that this has been corrected before. If anyone thinks that the synthesis should use iron(III) chloride, please check the literature and discuss any proposed change here. Thanks. EdChem (talk) 01:16, 4 December 2016 (UTC)
- Thank you for keeping an eye on these details. I will check, but my impression is that Pauson or maybe it was Woodward, both hard core organickers, was trying to oxidatively couple Cp- to make fulvalene, hence the use of Fe(III), even though the logical route to ferrocene is Fe(II). Fe(III) is and was a standard reagent for oxidative coupling. --Smokefoot (talk) 02:56, 4 December 2016 (UTC)
- @Smokefoot: you are, of course, absolutely correct. The goal was fulvalene and the use of iron(III) makes sense in that context. Sources (like this) confirm that they used FeCl
3. What puzzles me is what reduced the iron(III) to iron(II) with the CpMgBr to make Cp
2Fe. Oxidation of bromide should not be favourable and I would have thought the formation of bromine would be noted. Anyway, I need to change the article. Oops! EdChem (talk) 06:44, 4 December 2016 (UTC) - Addendum, of course, it is oxidation of cyclopentadienyl anion to dihydrofulvalene, which is what the equation I changed was trying to show. Anyway, found a good illustration. EdChem (talk) 07:42, 4 December 2016 (UTC)
- @Smokefoot: you are, of course, absolutely correct. The goal was fulvalene and the use of iron(III) makes sense in that context. Sources (like this) confirm that they used FeCl
- Thank you for keeping an eye on these details. I will check, but my impression is that Pauson or maybe it was Woodward, both hard core organickers, was trying to oxidatively couple Cp- to make fulvalene, hence the use of Fe(III), even though the logical route to ferrocene is Fe(II). Fe(III) is and was a standard reagent for oxidative coupling. --Smokefoot (talk) 02:56, 4 December 2016 (UTC)
SMILES and Jmol effects
I have altered the SMILES string into Jmol=[cH-]1cccc1.[Fe+2].[cH-]1cccc1I (for Jmol only)
May be incorrect by SMILES, but hey at least Jmol shows correct (Fe centered). -DePiep (talk) 23:39, 3 December 2016 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified one external link on Ferrocene. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20060505193757/http://www.osd.org.tr/14.pdf to http://www.osd.org.tr/14.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018.
{{source check- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 04:26, 30 September 2017 (UTC)
19612 citations to Fc and counting...
Ferrocene is a fun compound to discuss. Approximately 100 papers, reports, and patents appear per year on it. Which of these many thousand should be mentioned? --Smokefoot (talk) 01:30, 14 November 2018 (UTC)
Pauson / Kealy Diagram
I think that the Pauson / Kealy diagram in the article is flawed in that its stoichiometry is erroneous. I have stated a discussion at Wikipedia talk:WikiProject Chemistry#The Pauson/Kealy synthesis of ferrocene, to which all are invited to participate. I am also removing the image until the discussion is resolved. EdChem (talk) 02:56, 7 April 2019 (UTC)
- I've made some changes including adding a new diagram. EdChem (talk) 02:27, 16 April 2019 (UTC)

