Glypican 3
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) | |||||||||
| RefSeq (protein) | |||||||||
| Location (UCSC) | Chr X: 133.54 – 133.99 Mb | Chr X: 51.36 – 51.7 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
Glypican-3 is a protein that, in humans, is encoded by the GPC3 gene.[5][6][7][8] The GPC3 gene is located on human X chromosome (Xq26) where the most common gene (Isoform 2, GenBank Accession No.: NP_004475) encodes a 70-kDa core protein with 580 amino acids.[9] Three variants have been detected that encode alternatively spliced forms termed Isoforms 1 (NP_001158089), Isoform 3 (NP_001158090) and Isoform 4 (NP_001158091).[9]
Structure and function
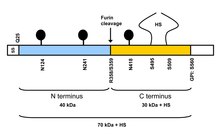
The protein core of GPC3 consists of two subunits, where the N-terminal subunit has a size of ~40 kDa and the C-terminal subunit is ~30 kDa.[9] Six glypicans (GPC1-6) have been identified in mammals. Cell surface heparan sulfate proteoglycans are composed of a membrane-associated protein core substituted with a variable number of heparan sulfate chains. Members of the glypican-related integral membrane proteoglycan family (GRIPS) contain a core protein anchored to the cytoplasmic membrane via a glycosyl phosphatidylinositol linkage. These proteins may play a role in the control of cell division and growth regulation.[7] GPC3 has been found to regulate Wnt/β-catenin and Yap signaling pathways.[9][10][11][12][13][14][15][16] GPC3 interacts with both Wnt and frizzled (FZD) to form a complex and triggers downstream signaling.[11][17] The core protein of GPC3 may serve as a co-receptor or a receiver for Wnt. A cysteine-rich domain at the N-lobe of GPC3 has been identified as a hydrophobic groove that interacts with Wnt3a.[17] Blocking the Wnt binding domain on GPC3 using the HN3 single domain antibody can inhibit Wnt activation.[17] Wnt also recognizes a heparan sulfate structure on GPC3 , which contains IdoA2S and GlcNS6S, and that the 3-O-sulfation in GlcNS6S3S significantly enhances the binding of Wnt to heparan sulfate.[10] GPC3 also modulates Yap signaling.[12] It might interact with FAT1 on the cell surface.[15]
Disease linkage
Deletion mutations in this gene are associated with Simpson–Golabi–Behmel syndrome.[5]
Diagnostic utility
Glypican 3 immunostaining has utility for differentiating hepatocellular carcinoma (HCC)[18] and dysplastic changes in cirrhotic livers; HCC stains with glypican 3, while liver with dysplastic changes and/or cirrhotic changes does not.[19] Using the YP7 murine monoclonal antibody, GPC3 protein expression is found in HCC, not in normal liver and cholangiocarcinoma.[20] The YP7 murine antibody has been humanized and named as 'hYP7'.[21] GPC3 is also expressed to a lesser degree in melanoma, ovarian clear-cell carcinomas, yolk sac tumors, neuroblastoma, hepatoblastoma, Wilms' tumor cells, and other tumors.[9] However, the significance of GPC3 as a diagnostic tool for human tumors other than HCC is unclear.[citation needed]
Therapeutic potential
To validate GPC3 as a therapeutic target in liver cancer, the anti-GPC3 therapeutic antibodies GC33,[22] YP7,[20] HN3[12] and HS20[13][23] have been made and widely tested. The laboratory of Mitchell Ho at the National Cancer Institute, NIH (Bethesda, Maryland, US) has generated YP7 and other murine monoclonal antibodies that recognize the C-lobe of GPC3 by hybridoma technology.[20] These antibodies have been humanized (e.g. hYP7) via antibody engineering for clinical applications.[21] The Ho lab has also identified the human single-domain antibody ('human nanobody') HN3[12] targeting the N-lobe of GPC3 [17] and the human monoclonal antibody HS20[13][24] targeting the heparan sulfate chains on GPC3 by phage display technology. Both HN3 and HS20 antibodies inhibit Wnt signaling in liver cancer cells . The immunotoxins based on HN3,[14][25][26] the antibody-drug conjugates based on hYP7[27] and the T-cell engaging bispecific antibodies derived from YP7[28][29] and GC33,[30] have been developed for treating liver cancer. The chimeric antigen receptor (CAR) T cell immunotherapies based on GC33,[31] hYP7[32][33] and HN3[34] are being developed at various stages for treating liver cancer. In mice with xenograft or orthoptic liver tumors, CAR (hYP7) T cells can eliminate GPC3-positive cancer cells, by inducing perforin- and granzyme-mediated cell death and reducing Wnt signaling in tumor cells.[33] CAR (hYP7) T cells are being evaluated at a clinical trial at the NIH.[35]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000147257 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000055653 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ S2CID 38846721.
- PMID 9787072.
- ^ a b "Entrez Gene: GPC3 glypican 3".
- PMID 17258707.
- ^ PMID 21112773.
- ^ PMID 27185050.
- ^ PMID 30352677.
- ^ PMID 23471984.
- ^ PMID 24492943.
- ^ PMID 25758784.
- ^ PMID 33420124.
- PMID 31428581.
- ^ PMID 30963603.
- S2CID 6312940.
- S2CID 45888415.
- ^ PMID 22820551.
- ^ PMID 27667400.
- PMID 19047163.
- PMID 30091851.
- PMID 30091851.
- PMID 27419635.
- PMID 31520528.
- PMID 30353932.
- ^ "Federal Register /Vol. 82, No. 96 / Friday, May 19, 2017" (PDF).
- PMID 34725191.
- S2CID 206693656.
- S2CID 24474000.
- S2CID 81043794.
- ^ PMID 32060001.
- PMID 36691969.
- ^ NCT05003895
Further reading
- Li M, Squire JA, Weksberg R (March 1998). "Overgrowth syndromes and genomic imprinting: from mouse to man". Clinical Genetics. 53 (3): 165–170. S2CID 85106528.
- Filmus J (March 2001). "Glypicans in growth control and cancer". Glycobiology. 11 (3): 19R–23R. PMID 11320054.
- Filmus J, Shi W, Wong ZM, Wong MJ (October 1995). "Identification of a new membrane-bound heparan sulphate proteoglycan". The Biochemical Journal. 311 (Pt 2): 561–565. PMID 7487896.
- Watanabe K, Yamada H, Yamaguchi Y (September 1995). "K-glypican: a novel GPI-anchored heparan sulfate proteoglycan that is highly expressed in developing brain and kidney". The Journal of Cell Biology. 130 (5): 1207–1218. PMID 7657705.
- Xuan JY, Besner A, Ireland M, Hughes-Benzie RM, MacKenzie AE (January 1994). "Mapping of Simpson-Golabi-Behmel syndrome to Xq25-q27". Human Molecular Genetics. 3 (1): 133–137. PMID 7909248.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–174. PMID 8125298.
- Shen T, Sonoda G, Hamid J, Li M, Filmus J, Buick RN, Testa JR (January 1997). "Mapping of the Simpson-Golabi-Behmel overgrowth syndrome gene (GPC3) to chromosome X in human and rat by fluorescence in situ hybridization". Mammalian Genome. 8 (1): 72. S2CID 9804496.
- Lage H, Dietel M (April 1997). "Cloning and characterization of human cDNAs encoding a protein with high homology to rat intestinal development protein OCI-5". Gene. 188 (2): 151–156. PMID 9133586.
- Huber R, Crisponi L, Mazzarella R, Chen CN, Su Y, Shizuya H, et al. (October 1997). "Analysis of exon/intron structure and 400 kb of genomic sequence surrounding the 5'-promoter and 3'-terminal ends of the human glypican 3 (GPC3) gene". Genomics. 45 (1): 48–58. PMID 9339360.
- Hsu HC, Cheng W, Lai PL (November 1997). "Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution". Cancer Research. 57 (22): 5179–5184. PMID 9371521.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–156. PMID 9373149.
- Pellegrini M, Pilia G, Pantano S, Lucchini F, Uda M, Fumi M, et al. (December 1998). "Gpc3 expression correlates with the phenotype of the Simpson-Golabi-Behmel syndrome". Developmental Dynamics. 213 (4): 431–439. S2CID 1966827.
- Huber R, Mazzarella R, Chen CN, Chen E, Ireland M, Lindsay S, et al. (December 1998). "Glypican 3 and glypican 4 are juxtaposed in Xq26.1". Gene. 225 (1–2): 9–16. PMID 9931407.
- Xuan JY, Hughes-Benzie RM, MacKenzie AE (January 1999). "A small interstitial deletion in the GPC3 gene causes Simpson-Golabi-Behmel syndrome in a Dutch-Canadian family". Journal of Medical Genetics. 36 (1): 57–58. PMID 9950367.
- Veugelers M, Cat BD, Muyldermans SY, Reekmans G, Delande N, Frints S, et al. (May 2000). "Mutational analysis of the GPC3/GPC4 glypican gene cluster on Xq26 in patients with Simpson-Golabi-Behmel syndrome: identification of loss-of-function mutations in the GPC3 gene". Human Molecular Genetics. 9 (9): 1321–1328. PMID 10814714.
- Khan S, Blackburn M, Mao DL, Huber R, Schlessinger D, Fant M (January 2001). "Glypican-3 (GPC3) expression in human placenta: localization to the differentiated syncytiotrophoblast". Histology and Histopathology. 16 (1): 71–78. PMID 11193214.
