Hydrazone
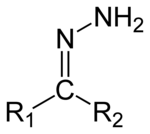
Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2.[1] They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.[2][3]
Synthesis
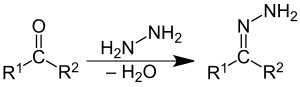
Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones.
Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides.[4][5]
Uses
Hydrazones are the basis for various analyses of ketones and aldehydes. For example,
The compound
.Hydrazones are the basis of
Reactions
Hydrazones are susceptible to hydrolysis:
- R2C=N−NR'2 + H2O → R2C=O + H2N−NR'2
When derived from hydrazine itself, hydrazones condense with a second
- R2C=N−NH2 + R2C=O → R2C=N−N=CR2 + H2O
Hydrazones are intermediates in the Wolff–Kishner reduction.
Hydrazones are reactants in
N,N-dialkylhydrazones
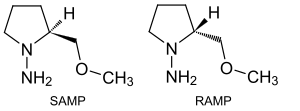
In N,N-dialkylhydrazones[15] the C=N bond can be hydrolysed, oxidised and reduced, the N–N bond can be reduced to the free amine. The carbon atom of the C=N bond can react with organometallic nucleophiles. The alpha-hydrogen atom is more acidic by 10 orders of magnitude compared to the ketone and therefore more nucleophilic. Deprotonation with for instance lithium diisopropylamide (LDA) gives an azaenolate which can be alkylated by alkyl halides.[16] The hydrazines SAMP and RAMP function as chiral auxiliary.[17][18]
Recovery of carbonyl compounds from N,N-dialkylhydrazones
Several methods are known to recover carbonyl compounds from N,N-dialkylhydrazones.[19] Procedures include oxidative, hydrolytic or reductive cleavage conditions and can be compatible with a wide range of functional groups.
Gallery
- Hydrazones
-
Benzophenone hydrazone, an illustrative hydrazone
-
Gyromitrin (acetaldehyde methylformylhydrazone), a toxin
-
Dihydralazine, an antihypertensive drug
-
X-ray structure of DNP-derived hydrazone of benzophenone. Selected parameters: C=N, 128 pm; N-N, 138 pm, N-N-C(Ar), 119 pm[20]
See also
References
- OCLC 642506595
- ^ Stork, G.; Benaim, J. (1977). "Monoalkylation of α,β-Unsaturated Ketones via Metalloenamines: 1-butyl-10-methyl-Δ1(9)-2-octalone". Organic Syntheses. 57: 69; Collected Volumes, vol. 6, p. 242.
- ^ Day, A. C.; Whiting, M. C. (1970). "Acetone hydrazone". Organic Syntheses. 50: 3; Collected Volumes, vol. 6, p. 10.
- .
- .
- PMID 28640998.
- PMID 21585205.
- S2CID 27226728.
- PMID 18712739.
- .
- ^ Lasri, Jamal; Ismail, Ali I. (2018). "Metal-free and FeCl3-catalyzed synthesis of azines and 3,5-diphenyl-1H-pyrazole from hydrazones and/or ketones monitored by high resolution ESI+-MS". Indian Journal of Chemistry, Section B. 57B (3): 362–373.
- .
- doi:10.3987/COM-13-12867 (inactive 2024-02-17).)
{{cite journal}}: CS1 maint: DOI inactive as of February 2024 (link - S2CID 11653420.
- PMID 20000672.
- .
- S2CID 260330996.
- .
- PMID 10727205.
- .





![X-ray structure of DNP-derived hydrazone of benzophenone. Selected parameters: C=N, 128 pm; N-N, 138 pm, N-N-C(Ar), 119 pm[20]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/39/NERYOZ.png/120px-NERYOZ.png)