Management of thalassemia
Treatment of the inherited blood disorder
Levels of severity
- Mild thalassemia : patients with thalassemia
- Severe thalassemia : Patients with severe thalassemia require medical treatment. A blood transfusion regimen was the first measure effective in prolonging life.[2]
Medication
Patients with thalassemia gradually accumulate high levels of iron (Fe) in their bodies. This build-up of iron may be due to the disease itself, from irregular hemoglobin not properly incorporating adequate iron into its structure, or it may be due to the many blood transfusions received by the patient. This overload of iron brings with it many biochemical complications.[citation needed]
Two key substances involved in iron transport and storage in the body are ferritin and transferrin. Ferritin is a protein present within cells that binds to Fe (II) and stores it as Fe (III), releasing it into the blood whenever required. Transferrin is an iron-binding protein present in blood plasma; transferrin acts as a transporter, carrying iron through blood and providing cells with the metal through endocytosis. Transferrin is highly specific to iron (III), and binds to it with an equilibrium constant of 1023 M−1 at a pH of 7.4.[5]
Thalassemia results in nontransferrin-bound iron being available in blood as all the transferrin becomes fully saturated. This free iron is toxic to the body since it catalyzes reactions that generate free hydroxyl radicals.[6] These radicals may induce lipid peroxidation of organelles like lysosomes, mitochondria, and sarcoplasmic membranes. The resulting lipid peroxides may interact with other molecules to form cross links, and thus either cause these compounds to perform their functions poorly, or render them non-functional altogether.[6] This iron overload may be treated with chelation therapy. Deferoxamine, deferiprone and deferasirox are the three most widely used iron-chelating agents.[citation needed]
Deferoxamine
Structure and coordination
The drug
Administration and action
Deferoxamine is administered via
Side effects
Deferoxamine could lead to toxic side effects if doses greater than 50 mg/kg body weight are administered. These side effects may include auditory and ocular abnormalities, pulmonary toxicity, sensorimotor neurotoxicity, as well as changes in renal function.[6] Another toxic effect of deferoxamine mostly observed in children is the failure of linear growth. This reduction in height may occur as a result of deferoxamine chelating metals other than iron which are required for normal growth. Deferoxamine has an
The toxic effect of deferoxamine on linear growth could also be due to excess deferoxamine accumulating in tissues and interfering with iron-dependent enzymes which are involved in the post-translational modification of collagen.[13]
Patients who receive
It has also been proven that combined treatment with deferoxamine and deferiprone leads to an increased efficiency in chelation and doubles iron excretion.[15]
Deferiprone
Structure and coordination
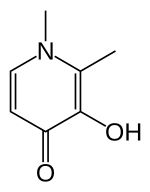
Deferiprone (DFP) is a
DFP is synthetically made and is highly selective to Fe(III).
Although the
Administration and action
Deferiprone is an iron chelator that is orally active, its administration thus being much easier than that for deferoxamine.[16] Plasma levels for the iron-drug complex climax after one hour of intake and the drug has a half-life of 160 minutes. Most of the iron-drug complex is therefore excreted within three to four hours following administration, the excretion occurring mostly in urine (90%).[16]
When comparing deferiprone to deferoxamine, they both bind iron with similar efficiency. However, drugs with different properties are able to access different iron pools. DFP is smaller than deferoxamine and can thus enter cells more easily. Also, at the pH of blood, the affinity of DFP for iron is concentration dependent: at low DFP concentrations, the iron-drug complex breaks down and the iron is donated to another competing ligand. This property accounts for the observed tendency of DFP to redistribute iron in the body. For the same reason, DFP can ‘shuttle’ intracellular iron out to the plasma, and transfer the iron to deferoxamine which goes on to expel it from the body.[17]
DFP was also found to be significantly more effective than deferoxamine in treating myocardial siderosis in patients with thalassemia major:[16] DFP is thought to improve the function of mitochondria in the heart by accessing and redistributing labile iron in cardiac cells.[citation needed]
Thalassemia patients may also be faced with potential oxidative damage to brain cells as the brain has high oxygen demands, but contains relatively low levels of antioxidant agents for protection against oxidation. The presence of excess iron in the brain may lead to higher concentrations of free radicals. Hexadentate chelators, like deferoxamine, are large molecules, and are thus unlikely to be able to cross the blood–brain barrier to chelate the excess iron. DFP, however, can do so and forms a soluble, neutral iron-drug complex that can cross cell membranes by non-facilitated diffusion. Attaching the drug to sugars may additionally enhance the penetration of the blood–brain barrier, as the brain uses facilitated transport for its relatively high levels of sugar intake.[20]
Side effects
DFP can be subjected to
Deferasirox
Structure and coordination

Deferasirox is an N-substituted bis-hydroxyphenyl-triazole. It is capable of removing iron from the blood through the coordination of two molecules of the deferasirox to a single iron ion, which forms the iron chelate (Fe-[deferasirox]2).
Administration and action
Deferasirox is most commonly marketed under the brand name Exjade. It has one key advantage over desferoxamine in that it can be taken orally in pill form, and so does not require
Side effects
Deferasirox can, however, have a wide variety of side effects. These may include headaches, nausea, vomiting, and joint pains.[25] Some evidence has been shown of a link to gastrointestinal disorders experienced by some people who have received the treatment.[24]
Indicaxanthin
Structure

Indicaxanthin is a pigment derived from the cactus pear fruit and can be used as an antioxidant. Dietary indicaxanthin has been shown to have protective effects on RBCs in people with beta thalassemia.[26] It has a structure similar to that of amino acids, and is amphiphilic: it is able to bind to cell membranes through charge-related interactions with polar head groups of membrane constituents, as well through adsorption to the lipid aggregates. Upon ex vivo introduction to thalassemic blood, indicaxanthin was shown to accumulate within RBCs.[26]
Function
Hb undergoes the following oxidation reaction during normal controlled breakdown of RBCs:[citation needed]
Hb → Oxy-Hb → Met-Hb → [Perferryl-Hb] → Oxoferryl → further oxidation steps
This reaction is experienced by thalassemic RBCs to a greater extent because, not only are there more oxidative radicals in thalassemic blood, but thalassemic RBCs also have limited antioxidant defense. Indicaxanthin is able to reduce the perferryl-Hb, a reactive intermediate, back to met-Hb. The overall effect of this step is that Hb degradation is prevented, which helps prevent accelerated breakdown of RBCs.[26]
In addition, indicaxathin has been shown to reduce oxidative damage in cells and tissues and does so by binding to radicals. The mechanism of its function, however, is still unknown.[26]
Indicaxanthin has high bioavailability and minimal side effects, like vomiting or diarrhea.[citation needed]
Carrier detection
- A screening policy exists in Cyprus to reduce the incidence of thalassemia, which since the program's implementation in the 1970s (which also includes pre-natal screening and abortion) has reduced the number of children born with the hereditary blood disease from 1 out of every 158 births to almost zero.[27]
- In Iran as a premarital screening, the man's red cell indices are checked first, if he has microcytosis (mean cell hemoglobin < 27 pg or mean red cell volume < 80 fl), the woman is tested. When both are microcytic their hemoglobin A2 concentrations are measured. If both have a concentration above 3.5% (diagnostic of thalassemia trait) they are referred to the local designated health post for genetic counseling.[28]
In 2008, in
Bone marrow transplant
It is possible to be cured, by a bone marrow transplantation (BMT) from compatible donor. In low-risk young people, the thalassemia-free survival rate is 87%; the mortality risk is 3%.[31] The drawback is that this method requires an HLA-matched compatible donor.[citation needed]
If the person does not have an HLA-matched compatible donor, there is another method called bone marrow transplantation from haploidentical mother to child (mismatched donor), in which the donor is the mother. The results are these: thalassemia-free survival rate 70%, rejection 23%, and mortality 7%. The best results are with very young people.[32]
References
- PMID 31529486.
- ^ a b "Pediatric Thalassemia Treatment & Management". Medical Care. Open Publishing. 30 April 2010. Retrieved 27 September 2011.
- ^ Claude Owen Burdick. "Separating Thalassemia Trait and Iron Deficiency by Simple Inspection". American Society for Clinical Pathology. Archived from the original on 22 September 2014. Retrieved 27 September 2011.
- ISBN 978-0-07-164114-2.
- PMID 204636.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - ^ PMID 9028304. Retrieved 28 February 2013.
- PMID 8047080.
- .
- PMID 7299539.
- S2CID 6802958.
- S2CID 34700148.
- PMID 8219368.
- PMID 1550263.
- PMID 7114394.
- S2CID 29566007.
- ^ PMID 17722270.
- ^ PMID 9028304.
- ^ S2CID 31161087.
- PMID 17722270.
- S2CID 41639654.
- PMID 16352812.
- PMID 17018383.
- S2CID 12396225.
- ^ PMID 21628399.
- ^ "How Are Thalassemias Treated?". National Heat, Lung and Blood institute. Retrieved March 2, 2013.
- ^ S2CID 25837703.
- S2CID 41877258.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - PMID 15539666.
- ^ Spanish Baby Engineered To Cure Brother
- Times of India, 17 September 2009
- PMID 21119108.
- PMID 22053275.
