Plumbagin
| |

| |
| Names | |
|---|---|
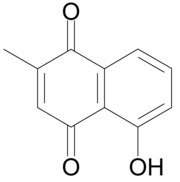
| Preferred IUPAC name
5-Hydroxy-2-methylnaphthalene-1,4-dione | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.006.882 |
IUPHAR/BPS |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H8O3 | |
| Molar mass | 188.17942 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Plumbagin or 5-hydroxy-2-methyl-1,4-naphthoquinone is an
mutagenic.[3]
Plumbagin is a yellow dye,[1] formally derived from naphthoquinone.
It is named after the plant genus Plumbago, from which it was originally isolated.[4] It is also commonly found in the carnivorous plant genera Drosera and Nepenthes.[5][6] It is also a component of the black walnut drupe.
See also
References
- ^ a b Black Walnut. Drugs.com.
- PMID 19124069.
- PMID 2933393.
- .
- ^ Wang, W.; Luo, X.; Li, H. (2010). "Terahertz and Infrared Spectra of Plumbagin, Juglone, and Menadione". Carnivorous Plant Newsletter. 39 (3): 82–88.
- PMID 11867092.
