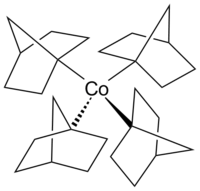
Tetrakis(1-norbornyl)cobalt(IV)
| |
| Names | |
|---|---|
| Other names
(T-4)-Tetrakis(bicyclo[2.2.1]hept-1-yl)cobalt
| |
| Identifiers | |
3D model (
JSmol ) |
|
| |
| |
| Properties | |
| C28H44Co | |
| Molar mass | 439.593 g·mol−1 |
| Appearance | brown crystals |
| Melting point | 100 °C (decomposes) |
| Solubility | soluble in THF |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrakis(1-norbornyl)cobalt(IV) is an air-sensitive organometallic compound of cobalt. It was first synthesized by Barton K. Bower and Howard G. Tennent in 1972[1] and is one of few compounds in which cobalt has a formal oxidation state of +4.
Preparation
Tetrakis(1-norbornyl)cobalt(IV) is formed the reaction of CoCl2•THF with 1-norbornyllithium (norLi) in
The compound can then be purified by recrystallization.
Properties
The complex is a thermally stable
Stability
The exceptional stability of the complex is in large part due to its inability to undergo either α- or β-hydride elimination. The α-position of the metal (corresponding to the 1-position of the norbornyl ligand) has no more hydrogen atoms, while hydride elimination from the β-position would yield an energetically unfavorable double bond on a bridgehead atom (Bredt's rule). Moreover, the bulky norbornyl ligands sterically shield the central atom, hindering ligand substitutions as well as homolysis.[1][5]
The rare d5 low-spin configuration in a tetrahedral ligand field is possible because the ligand is so strongly σ-donating that the gap between the e und t2 orbitals is raised sufficiently to overcome the spin pairing energy. The resulting configuration is e4t21, with magnetic measurements showing paramagnetism consistent with only one unparied electron.[1][3][4]
Cobalt(III) and cobalt(V) derivatives
The reaction between CoCl2•THF and 1-norbornyllithium (norLi) also allows the formation of a cobalt(III) complex: if a mixture of diethyl ether and THF is used as the solvent in place of n-pentane, the resulting disproportionation reaction affords the complex tetrakis(1-norbornyl)cobaltate(III), which crystallizes out of solution with solvated lithium counterions, along with elemental cobalt.[4][6]
The compound is air-sensitive, has a green color and is
The corresponding cobalt(V) complex is prepared by oxidizing tetrakis(1-norbornyl)cobalt(IV) with Ag[BF4] in THF and crystallizes with tetrafluoroborate as the counterion.[6][4]
- Co(nor)4 + AgBF4 → [Co(nor)4]BF4 + Ag
This complex :[Co(nor)4]+ is the first cobalt(V) complex to be isolated. Again the configuration is low-spin (d4, e4t20).[2][4][6]
See also
References
- ^ .
- ^ )
- ^ .
- ^ .
- ISBN 978-3-11-020685-2.
- ^ .
External links
- Bower, Barton K. (12 December 1972). "Tetra(bicycloheptyl) transition metal compounds".

![{\displaystyle {\ce {{2CoCl2.THF}+4norLi->[{\text{pentane}}][]{[Co(nor)4]}+{Co}+4{LiCl}+2THF}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a37098ecef41ed86567264cfd41e5f41268dae1b)
![{\displaystyle {\ce {3{CoCl2.THF}+8{norLi}+5{THF}->[{} \atop {\ce {Et2O/THF}}]2{[Li(THF)4][Co(nor)4]}+{Co}+LiCl}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c58c9aa237931bf20327128ad7a588b8bf9cac82)