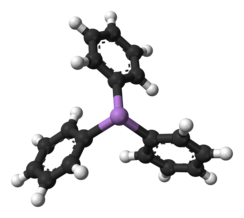
Triphenylarsine
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Triphenylarsane | |
| Other names
Tribenzenidoarsenic
Triphenylarsine | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.009.121 |
| EC Number |
|
PubChem CID
|
|
RTECS number
|
|
| UNII | |
| UN number | 3465 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H15As | |
| Molar mass | 306.240 g·mol−1 |
| Appearance | Colourless solid |
| Density | 1.395 g cm−3 |
| Melting point | 58 to 61 °C (136 to 142 °F; 331 to 334 K) |
| Boiling point | 373 °C (703 °F; 646 K) at 760 mmHg |
| Insoluble | |
| Solubility | Soluble in ethyl ether, benzene, slightly soluble in ethanol |
| -177.0·10−6 cm3/mol | |
| Structure | |
| Triclinic | |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H301, H331, H410 | |
| P261, P264, P270, P271, P273, P301+P310, P304+P340, P311, P321, P330, P391, P403+P233, P405, P501 | |
| Related compounds | |
Related organoarsanes
|
Trimethylarsine |
Related compounds
|
Triphenylamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triphenylarsine is the
coordination chemistry and organic synthesis. The molecule is pyramidal with As-C distances of 1.942–1.956 Å and C-As-C angles of 99.6–100.5°.[1]
This compound is prepared by the reaction of arsenic trichloride with chlorobenzene using sodium as the reducing agent:[2]
- AsCl3 + 3 PhCl + 6 Na → AsPh3 + 6 NaCl
Reactions
Reaction of triphenylarsine with lithium gives lithium diphenylarsenide:[3]
- AsPh3 + 2 Li → LiAsPh2 + LiPh
Triphenylarsine is the precursor to tetraphenylarsonium chloride, [AsPh4]Cl, a popular precipitating agent.[2]
AsPh3 forms
metal complexes with metals. Most are analogues of the corresponding triphenylphosphine derivatives. Examples include [[IrCl(CO)(AsPh3)]]2, [[RhCl(AsPh3)3]], and [[Fe(CO)4(AsPh3)]].[4]
Tetraphenylarsonium chloride is prepared from triphenylarsine:[5]
- (C6H5)3As + Br2 → (C6H5)3AsBr2
- (C6H5)3AsBr2 + H2O → (C6H5)3AsO + 2 HBr
- (C6H5)3AsO + C6H5MgBr → (C6H5)4AsOMgBr
- (C6H5)4AsOMgBr + 3 HCl → (C6H5)4AsCl.HCl + MgBrCl
- (C6H5)4AsCl.HCl + NaOH → (C6H5)4AsCl + NaCl + H2O
References
- ^ a b Shriner, R. L.; Wolf, C. N. (1963). "Tetraphenylarsonium Chloride Hydrochloride". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 910. article - ISBN 978-0-470-13247-0.
- ISBN 0-470-58117-4.
- .
