Water model

In
An alternative to the explicit water models is to use an implicit solvation model, also termed a continuum model, an example of which would be the COSMO solvation model or the polarizable continuum model (PCM) or a hybrid solvation model.[1]
Simple water models
The rigid models are considered the simplest water models and rely on non-bonded interactions. In these models, bonding interactions are implicitly treated by holonomic constraints. The electrostatic interaction is modeled using Coulomb's law, and the dispersion and repulsion forces using the Lennard-Jones potential.[2][3] The potential for models such as TIP3P (transferable intermolecular potential with 3 points) and TIP4P is represented by
where kC, the
The figure below shows the general shape of the 3- to 6-site water models. The exact geometric parameters (the OH distance and the HOH angle) vary depending on the model.
2-site
A 2-site model of water based on the familiar three-site SPC model (see below) has been shown to predict the dielectric properties of water using site-renormalized molecular fluid theory.[7]
3-site
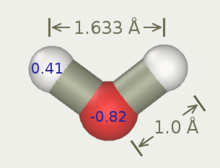
Three-site models have three interaction points corresponding to the three atoms of the water molecule. Each site has a point charge, and the site corresponding to the oxygen atom also has the Lennard-Jones parameters. Since 3-site models achieve a high computational efficiency, these are widely used for many applications of molecular dynamics simulations. Most of the models use a rigid geometry matching that of actual water molecules. An exception is the SPC model, which assumes an ideal tetrahedral shape (HOH angle of 109.47°) instead of the observed angle of 104.5°.
The table below lists the parameters for some 3-site models.
| TIPS[8] | SPC[9] | TIP3P[10] | SPC/E[11] | |
|---|---|---|---|---|
| r(OH), Å | 0.9572 | 1.0 | 0.9572 | 1.0 |
| HOH, deg | 104.52 | 109.47 | 104.52 | 109.47 |
| A, 103 kcal Å12/mol | 580.0 | 629.4 | 582.0 | 629.4 |
| B, kcal Å6/mol | 525.0 | 625.5 | 595.0 | 625.5 |
| q(O) | −0.80 | −0.82 | −0.834 | −0.8476 |
| q(H) | +0.40 | +0.41 | +0.417 | +0.4238 |
The SPC/E model adds an average polarization correction to the potential energy function:
where μ is the electric dipole moment of the effectively polarized water molecule (2.35 D for the SPC/E model), μ0 is the dipole moment of an isolated water molecule (1.85 D from experiment), and αi is an isotropic polarizability constant, with a value of 1.608×10−40 F·m2. Since the charges in the model are constant, this correction just results in adding 1.25 kcal/mol (5.22 kJ/mol) to the total energy. The SPC/E model results in a better density and diffusion constant than the SPC model.
The TIP3P model implemented in the CHARMM force field is a slightly modified version of the original. The difference lies in the Lennard-Jones parameters: unlike TIP3P, the CHARMM version of the model places Lennard-Jones parameters on the hydrogen atoms too, in addition to the one on oxygen. The charges are not modified.[12] Three-site model (TIP3P) has better performance in calculating specific heats.[13]
Flexible SPC water model
The flexible simple point-charge water model (or flexible SPC water model) is a re-parametrization of the three-site SPC water model.
Flexible SPC is implemented in the programs MDynaMix and Abalone.
Other models
- Ferguson (flexible SPC)[17]
- CVFF (flexible)
- MG (flexible and dissociative)[18]
- KKY potential (flexible model).[19]
- BLXL (smear charged potential).[20]
4-site
The four-site models have four interaction points by adding one dummy atom near of the oxygen along the
The TIP4P model, first published in 1983, is widely implemented in computational chemistry software packages and often used for the simulation of biomolecular systems. There have been subsequent reparameterizations of the TIP4P model for specific uses: the TIP4P-Ew model, for use with Ewald summation methods; the TIP4P/Ice, for simulation of solid water ice; TIP4P/2005, a general parameterization for simulating the entire phase diagram of condensed water; and TIP4PQ/2005, a similar model but designed to accurately describe the properties of solid and liquid water when quantum effects are included in the simulation.[22]
Most of the four-site water models use an OH distance and HOH angle which match those of the free water molecule. One exception is the OPC model, in which no geometry constraints are imposed other than the fundamental C2v molecular symmetry of the water molecule. Instead, the point charges and their positions are optimized to best describe the electrostatics of the water molecule. OPC reproduces a comprehensive set of bulk properties more accurately than several of the commonly used rigid n-site water models. The OPC model is implemented within the AMBER force field.
| BF[21] | TIPS2[23] | TIP4P[10] | TIP4P-Ew[24] | TIP4P/Ice[25] | TIP4P/2005[26] | OPC[27] | TIP4P-D[28] | |
|---|---|---|---|---|---|---|---|---|
| r(OH), Å | 0.96 | 0.9572 | 0.9572 | 0.9572 | 0.9572 | 0.9572 | 0.8724 | 0.9572 |
| HOH, deg | 105.7 | 104.52 | 104.52 | 104.52 | 104.52 | 104.52 | 103.6 | 104.52 |
| r(OM), Å | 0.15 | 0.15 | 0.15 | 0.125 | 0.1577 | 0.1546 | 0.1594 | 0.1546 |
| A, 103 kcal Å12/mol | 560.4 | 695.0 | 600.0 | 656.1 | 857.9 | 731.3 | 865.1 | 904.7 |
| B, kcal Å6/mol | 837.0 | 600.0 | 610.0 | 653.5 | 850.5 | 736.0 | 858.1 | 900.0 |
| q(M) | −0.98 | −1.07 | −1.04 | −1.04844 | −1.1794 | −1.1128 | −1.3582 | −1.16 |
| q(H) | +0.49 | +0.535 | +0.52 | +0.52422 | +0.5897 | +0.5564 | +0.6791 | +0.58 |
Others:
5-site
The 5-site models place the negative charge on dummy atoms (labelled L) representing the lone pairs of the oxygen atom, with a tetrahedral-like geometry. An early model of these types was the BNS model of Ben-Naim and Stillinger, proposed in 1971,[citation needed] soon succeeded by the ST2 model of Stillinger and Rahman in 1974.[31] Mainly due to their higher computational cost, five-site models were not developed much until 2000, when the TIP5P model of Mahoney and Jorgensen was published.[32] When compared with earlier models, the TIP5P model results in improvements in the geometry for the water dimer, a more "tetrahedral" water structure that better reproduces the experimental radial distribution functions from neutron diffraction, and the temperature of maximal density of water. The TIP5P-E model is a reparameterization of TIP5P for use with Ewald sums.
| BNS[31] | ST2[31] | TIP5P[32] | TIP5P-E[33] | |
|---|---|---|---|---|
| r(OH), Å | 1.0 | 1.0 | 0.9572 | 0.9572 |
| HOH, deg | 109.47 | 109.47 | 104.52 | 104.52 |
| r(OL), Å | 1.0 | 0.8 | 0.70 | 0.70 |
| LOL, deg | 109.47 | 109.47 | 109.47 | 109.47 |
| A, 103 kcal Å12/mol | 77.4 | 238.7 | 544.5 | 554.3 |
| B, kcal Å6/mol | 153.8 | 268.9 | 590.3 | 628.2 |
| q(L) | −0.19562 | −0.2357 | −0.241 | −0.241 |
| q(H) | +0.19562 | +0.2357 | +0.241 | +0.241 |
| RL, Å | 2.0379 | 2.0160 | ||
| RU, Å | 3.1877 | 3.1287 |
Note, however, that the BNS and ST2 models do not use Coulomb's law directly for the electrostatic terms, but a modified version that is scaled down at short distances by multiplying it by the switching function S(r):
Thus, the RL and RU parameters only apply to BNS and ST2.
6-site
Originally designed to study water/ice systems, a 6-site model that combines all the sites of the 4- and 5-site models was developed by Nada and van der Eerden.[34] Since it had a very high melting temperature[35] when employed under periodic electrostatic conditions (Ewald summation), a modified version was published later[36] optimized by using the Ewald method for estimating the Coulomb interaction.
Other
- The effect of explicit solute model on solute behavior in biomolecular simulations has been also extensively studied. It was shown that explicit water models affected the specific solvation and dynamics of unfolded peptides, while the conformational behavior and flexibility of folded peptides remained intact.[37]
- MB model. A more abstract model resembling the Mercedes-Benz logo that reproduces some features of water in two-dimensional systems. It is not used as such for simulations of "real" (i.e., three-dimensional) systems, but it is useful for qualitative studies and for educational purposes.[38]
- Coarse-grained models. One- and two-site models of water have also been developed.[39] In coarse-grain models, each site can represent several water molecules.
- Many-body models. Water models built using training-set configurations solved quantum mechanically, which then use machine learning protocols to extract potential-energy surfaces. These potential-energy surfaces are fed into MD simulations for an unprecedented degree of accuracy in computing physical properties of condensed phase systems.[40]
Computational cost
The computational cost of a water simulation increases with the number of interaction sites in the water model. The CPU time is approximately proportional to the number of interatomic distances that need to be computed. For the 3-site model, 9 distances are required for each pair of water molecules (every atom of one molecule against every atom of the other molecule, or 3 × 3). For the 4-site model, 10 distances are required (every charged site with every charged site, plus the O–O interaction, or 3 × 3 + 1). For the 5-site model, 17 distances are required (4 × 4 + 1). Finally, for the 6-site model, 26 distances are required (5 × 5 + 1).
When using rigid water models in molecular dynamics, there is an additional cost associated with keeping the structure constrained, using
See also
- Water (properties)
- Water (data page)
- Water dimer
- Force field (chemistry)
- Comparison of force field implementations
- Molecular mechanics
- Molecular modelling
- Comparison of software for molecular mechanics modeling
- Solvent models
References
- PMID 25660403.
- ISBN 978-0-19-855645-9.
- ^ Kirby BJ. Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices.
- ^ Swails JM, Roitberg AE (2013). "prmtop file of {A}mber" (PDF).
- ^ Swails JM (2013). Free energy simulations of complex biological systems at constant pH (PDF). University of Florida.
- ^ Case DA, Walker RC, Cheatham III TE, Simmerling CL, Roitberg A, Merz KM, et al. (April 2019). "Amber 2019 reference manual (covers Amber18 and AmberTools19)" (PDF).
- PMID 19920881.
- ISSN 0002-7863.
- ^ H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, and J. Hermans, In Intermolecular Forces, edited by B. Pullman (Reidel, Dordrecht, 1981), p. 331.
- ^ doi:10.1063/1.445869.
- .
- PMID 24889800.
- .
- ^
Toukan K, Rahman A (March 1985). "Molecular-dynamics study of atomic motions in water". Physical Review B. 31 (5): 2643–2648. PMID 9936106.
- ^ Berendsen HJ, Grigera JR, Straatsma TP (1987). "The missing term in effective pair potentials". .
- ^ Praprotnik M, Janezic D, Mavri J (2004). "Temperature Dependence of Water Vibrational Spectrum: A Molecular Dynamics Simulation Study". .
- S2CID 206038409. Retrieved 28 July 2021.
- ^ MG model Archived 2016-03-04 at the Wayback Machine.
- ISSN 0892-7022.
- doi:10.1063/1.478797.
- ^ .
- S2CID 15505037.
- doi:10.1063/1.444325.
- S2CID 39545298.
- S2CID 8382245.
- S2CID 9757894.
- PMID 25400877.
- PMID 25764013.
- S2CID 9095938.
- PMID 22168712.
- ^ S2CID 96035805.
- ^ S2CID 16367148.
- PMID 15267492.
- .
- S2CID 33883071.
- PMID 28049310.
- PMID 26617103.
- .
- PMID 16223273.
- PMID 26579763.
- PMID 27186804.
- S2CID 15215071.





