Acetal

In
The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of an R on the central carbon).[1] The IUPAC originally deprecated the usage of the word ketal altogether, but has since reversed its decision. However, in contrast to historical usage, ketals are now a subset of acetals, a term that now encompasses both aldehyde- and ketone-derived structures.
If one of the R groups has an oxygen as the first atom (that is, there are more than two oxygens single-bonded to the central carbon), the functional group is instead an
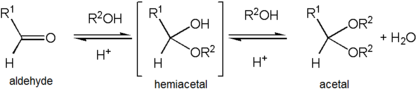
Formation of an acetal occurs when the
Acetals are stable compared to hemiacetals but their formation is a reversible
Acetalisation and ketalization
Acetalisation and ketalization are the organic reactions that involve the formation of an acetal (or ketals) from aldehydes and ketones, respectively. These conversions are acid catalysed. They eliminate water. Since each step is often a rapid equilibrium, the reaction must be driven by removal of water. Methods for removing water include azeotropic distillation and trapping water with desiccants like aluminium oxide and molecular sieves. Steps assumed to be involved: protonation of the carbonyl oxygen, addition of the alcohol to the protonated carbonyl, protonolysis of the resulting hemiacetal or hemiketal, and addition of the second alcohol. These steps are illustrated with an aldehyde RCH=O and the alcohol R'OH:
- RCH=O + H+ ⇌ RCH=OH+
- RCH=OH+ + R'OH ⇌ RCH(OH)(OR') + H+
- RCH(OH)(OR') + H+ ⇌ RC+H(OR') + H2O
- RC+H(OR') + R'OH ⇌ RCH(OR')2 + H+
Another way to avoid the entropic cost is to perform the synthesis by acetal exchange, using a pre-existing acetal-type reagent as the OR'-group donor rather than simple addition of alcohols themselves. One type of reagent used for this method is an orthoester. In this case, water produced along with the acetal product is destroyed when it hydrolyses residual orthoester molecules, and this side reaction also produces more alcohol to be used in the main reaction.
Examples
Sugars
Since many sugars are polyhydroxy aldehydes and ketones, sugars are a rich source of acetals and ketals. Most glycosidic bonds in carbohydrates and other polysaccharides are acetal linkages.[2] Cellulose is a ubiquitous example of a polyacetal.
Benzylidene acetal and acetonide as protecting groups used in research of modified sugars.
Chiral derivatives
Acetals also find application as chiral auxiliaries. Indeed acetals of chiral glycols like, e.g. derivatives of tartaric acid can be asymmetrically opened with high selectivity. This enables the construction of new chiral centers.[3]

Formaldehyde and acetaldehyde
Formaldehyde forms a rich collection of acetals. This tendency reflects the fact that low molecular weight aldehydes are prone to self-condensation such that the C=O bond is replaced by an acetal. The acetal formed from formaldehyde (two hydrogens attached to the central carbon) is sometimes called a formal[4] or the methylenedioxy group. The acetal formed from acetone is sometimes called an acetonide. Formaldehyde forms Paraldehyde and 1,3,5-Trioxane. Polyoxymethylene (POM) plastic, also known as "acetal" or "polyacetal", is a polyacetal (and a polyether), and a polymer of formaldehyde. Acetaldehyde converts to Metaldehyde.
Unusual acetals
Phenylsulfonylethylidene (PSE) acetal is an example of arylsulfonyl acetal possessing atypical properties, like resistance to acid hydrolysis which leads to selective introduction and removal of the protective group.[5]
Flavors and fragrances
Related compounds
Used in a more general sense, the term X,Y-acetal also refers to any functional group that consists of a carbon bearing two heteroatoms X and Y. For example, N,O-acetal refers to compounds of type R1R2C(OR)(NR'2) (R,R' ≠ H) also known as a hemiaminal ether or Aminal, a.k.a. aminoacetal.
S,S-acetal refers to compounds of type R1R2C(SR)(SR') (R,R' ≠ H, also known as thioacetal and thioketals.
See also
- Hemiaminal
- Orthoformate
References
- ^ P.J. Kocieński: Protecting Groups, S. 164–167.
- ^ Morrison, Robert T. and Boyd, Robert N., "Organic Chemistry (6th ed)". p683. Prentice-Hall Inc (1992).
- ISSN 0040-4039.
- ISBN 978-0-8247-8390-7.
- ISBN 978-3-527-30673-2.