Coordination sphere
In
First coordination sphere
The first coordination sphere refers to the molecules that are attached directly to the metal. The interactions between the first and second coordination spheres usually involve hydrogen-bonding. For charged complexes,
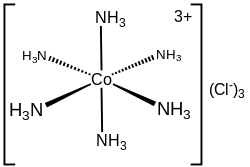
In
Second coordination sphere

Metal ions can be described as consisting of series of two concentric coordination spheres, the first and second. More distant from the second coordination sphere, the solvent molecules behave more like "bulk solvent." Simulation of the second coordination sphere is of interest in computational chemistry. The second coordination sphere can consist of ions (especially in charged complexes), molecules (especially those that hydrogen bond to ligands in the first coordination sphere) and portions of a ligand backbone. Compared to the first coordination sphere, the second coordination sphere has a less direct influence on the reactivity and chemical properties of the metal complex. Nonetheless the second coordination sphere is relevant to understanding reactions of the metal complex, including the mechanisms of ligand exchange and catalysis.
Role in catalysis
Mechanisms of metalloproteins often invoke modulation of the second coordination sphere by the protein.[1]

Role in mechanistic inorganic chemistry
The rates at which ligands exchange between the first and the second coordination sphere is the first step in ligand substitution reactions. In
The energetics of
- [Fe*(H2O)6]2+ + [Fe(H2O)5(OH)]2+ → [Fe(H2O)6]3+ + [Fe*(H2O)5(OH)]2+
Role in spectroscopy
Solvent effects on colors and stability are often attributable to changes in the second coordination sphere. Such effects can be pronounced in complexes where the ligands in the first coordination sphere are strong hydrogen-bond donors and acceptors, e.g. respectively [Co(NH3)6]3+ and [Fe(CN)6]3−. Crown-ethers bind to polyamine complexes through their second coordination sphere. Polyammonium cations bind to the nitrogen centres of cyanometallates.[5]
Role in supramolecular chemistry
Macrocyclic molecules such as cyclodextrins act often as the second coordination sphere for metal complexes. [6][7]
