Digital pathology
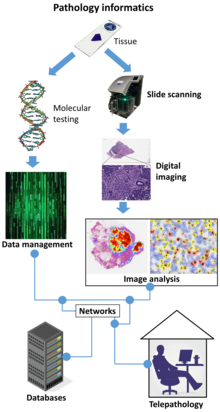
Digital pathology is a sub-field of pathology that focuses on data management based on information generated from digitized specimen slides. Through the use of computer-based technology, digital pathology utilizes virtual microscopy.[1] Glass slides are converted into digital slides that can be viewed, managed, shared and analyzed on a computer monitor. With the practice of whole-slide imaging (WSI), which is another name for virtual microscopy,[2] the field of digital pathology is growing and has applications in diagnostic medicine, with the goal of achieving efficient and cheaper diagnoses, prognosis, and prediction of diseases due to the success in machine learning and artificial intelligence in healthcare.[3]
History
The roots of digital pathology go back to the 1960s, when first telepathology experiments took place. Later in the 1990s the principle of virtual microscopy[4] appeared in several life science research areas. At the turn of the century the scientific community more and more agreed on the term "digital pathology" to denote digitization efforts in pathology. However, in 2000, the technical requirements (scanner, storage, network) were still a limiting factor for a broad dissemination of digital pathology concepts. This changed as new powerful and affordable scanner technology as well as mass / cloud storage technologies appeared on the market. The field of radiology has undergone the digital transformation almost 15 years ago, not because radiology is more advanced, but there are fundamental differences between digital images in radiology and digital pathology: The image source in radiology is the (alive) patient, and today in most cases, the image is even primarily captured in digital format. In pathology the scanning is done from preserved and processed specimens, for retrospective studies even from slides stored in a biobank. Besides this difference in pre-analytics and metadata content, the required storage in digital pathology is two to three orders of magnitude higher than in radiology. However, the advantages anticipated through digital pathology are similar to those in radiology:
- Capability to transmit digital slides over distances quickly, which enables telepathology scenarios.
- Capability to access past specimen from the same patients and similar cases for comparison and review, with much less effort than retrieving slides from the archive shelves.
- Capability to compare different areas of multiple slides simultaneously (slide by slide mode) with the help of a virtual microscope.
- Capability to annotate areas directly in the slide and share this for teaching and research.
Digital pathology is today widely used for educational purposes
Environment
Scan


Digital slides are created from glass slides using specialized scanning machines. All high quality scans must be free of dust, scratches, and other obstructions. There are two common methods for digital slide scanning, tile-based scanning and line-based scanning.[6] Both technologies use an integrated camera and a motorized stage to move the slide around while parts of the tissue are imaged. Tile scanners capture square field-of-view images covering the entire tissue area on the slide, while line-scanners capture images of the tissue in long, uninterrupted stripes rather than tiles. In both cases, software associated with the scanner stitch the tiles or lines together into a single, seamless image.
Z-stacking is the scanning of a slide at multiple focal planes along the vertical z-axis.[7]
View
Digital slides are accessible for viewing via a computer monitor and viewing software either locally or remotely via the Internet. An example of an
bindings are also available) that provides a simple interface to read and view whole-slide images.Manage
Digital slides are maintained in an information management system that allows for archival and intelligent retrieval.
Network
Digital slides are often stored and delivered over the Internet or private networks, for viewing and consultation.
Analyze
Image analysis tools are used to derive objective quantification measures from digital slides.
-
Ki67 stain calculation by QuPath in a pure seminoma, which gives a measure of the proliferation rate of the tumor. The colors represent the intensity of expression: blue-no expression, yellow-low, orange-moderate, and red-high expression.[14]
-
Tissue segmentation for digital calculation of bone marrow cellularity in QuPath: The system is trained on the appearance of immune cells versus other tissue, and uses this to give an overall percentage of each type.
-
Breast cancer prediction by AI.[15]
Integrate
Digital pathology workflow is integrated into the institution's overall operational environment. Slide digitization is expected to reduce the number of routine, manually reviewed slides, maximizing workload efficiency.
Sharing
Digital pathology also allows internet information sharing for education, diagnostics, publication and research. This may take the form of publicly available datasets or open source access to
Challenges


Digital pathology has been approved by the FDA for primary diagnosis.[16] The approval was based on a multi-center study of 1,992 cases in which whole-slide imaging (WSI) was shown to be non-inferior to microscopy across a wide range of surgical pathology specimens, sample types and stains.[17] While there are advantages to WSI when creating digital data from glass slides, when it comes to real-time telepathology applications, WSI is not a strong choice for discussion and collaboration between multiple remote pathologists.[18] Furthermore, unlike digital radiology where the elimination of film made return on investment (ROI) clear, the ROI on digital pathology equipment is less obvious. The strongest ROI justification includes improved quality of healthcare, increased efficiency for pathologists, and reduced costs in handling glass slides.[19]
Validation
Validation of a digital microscopy workflow in a specific environment (see above) is important to ensure high diagnostic performance of pathologists when evaluating digital whole-slide images. There are different methods that can be used for this validation process.[20] The College of American Pathologists has published a guideline with minimal requirements for validation of whole slide imaging systems for diagnostic purposes in human pathology.[21]
Potential
Trained pathologists traditionally view tissue slides under a microscope. These tissue slides may be stained to highlight cellular structures. When slides are digitized, they are able to be shared through tele-pathology and are numerically analyzed using computer algorithms. Algorithms can be used to automate the manual counting of structures, or for classifying the condition of tissue such as is used in grading tumors. They can additionally be used for feature detection of mitotic figures, epithelial cells, or tissue specific structures such as lung cancer nodules, glomeruli, or vessels, or estimation of molecular biomarkers such as mutated genes, tumor mutational burden, or transcriptional changes.[22][23][24] This has the potential to reduce human error and improve accuracy of diagnoses. Digital slides can be easily shared, increasing the potential for data usage in education as well as in consultations between expert pathologists. Multiplexed imaging (staining multiple markers on the same slide) allows pathologists to understand finer distribution of cell-types and their relative locations.[25] An understanding of the spatial distribution of cell-types or markers and pathways they express, can allow for prescription of targeted drugs or build combinational therapies in a personalized manner.
See also
References
- PMID 30607307.
- ^ "Whole Slide Imaging | MBF Bioscience". www.mbfbioscience.com. Retrieved 2019-12-02.
- PMID 37932267.
- PMID 9357666.
- S2CID 20599493.
- ^ "Informatics, digital & computational pathology".
- PMID 28440660.
- ^ OpenSeadragon on GitHub
- ^ QuPath on GitHub
- ^ OpenSlide website
- PMID 23355955.
- ^ "Geometric Performance Primitives (GPP)". NVIDIA Developer.
- ^ "Letter to Kaibo Wang" (PDF).
- PMID 28418027.
"This work is licensed under a Creative Commons Attribution 4.0 International License." - ^ "FDA allows marketing of first whole slide imaging system for digital pathology" (Press release). FDA. April 12, 2017. Retrieved May 24, 2017.
- PMID 28961557.
- PMID 30393436.
- ^ "How to Build a Business Case to Justify the Investment in Digital Pathology". Sectra Medical Systems. Retrieved April 26, 2015.
- PMID 34433345.
- PMID 34003251.
- PMID 30984469.
- S2CID 220510782.
- PMID 31632973.
- PMID 35404441.
Further reading
- Kayser, K; Kayser, G; Radziszowski, D; Oehmann, A (1999). "From telepathology to virtual pathology institution: The new world of digital pathology" (PDF). Romanian Journal of Morphology and Embryology. 45: 3–9. PMID 15847374.
- McCullough, Bruce; Ying, Xiaoyou; Monticello, Thomas; Bonnefoi, Marc (2004). "Digital Microscopy Imaging and New Approaches in Toxicologic Pathology". Toxicologic Pathology. 32 (5): 49–58. PMID 15503664.
- Schlangen, David; Stede, Manfred; Bontas, Elena Paslaru (2004). "Feeding OWL: Extracting and Representing the Content of Pathology Reports". NLPXML '04 Proceedings of the Workshop on NLP and XML. Nlpxml '04: 43–50.
- Cruz-Roa, Angel; Díaz, Gloria; Romero, Eduardo; González, Fabio (2011). "Automatic Annotation of Histopathological Images Using a Latent Topic Model Based On Non-negative Matrix Factorization". Journal of Pathology Informatics. 2 (4): 4. PMID 22811960.
- "E-Health and Telemedicine". International Journal of Computer Assisted Radiology and Surgery. 1 (Supplement 1): 119–35. 2006. S2CID 5642084.
- Fine, Jeffrey L.; Grzybicki, Dana M.; Silowash, Russell; Ho, Jonhan; Gilbertson, John R.; Anthony, Leslie; Wilson, Robb; Parwani, Anil V.; et al. (2008). "Evaluation of whole slide image immunohistochemistry interpretation in challenging prostate needle biopsies". Human Pathology. 39 (4): 564–72. PMID 18234276.
- Kayser, Klaus; Kayser, Gian; Radziszowski, Dominik; Oehmann, Alexander (2004). "New Developments in Digital Pathology: from Telepathology to Virtual Pathology Laboratory". In Duplaga, Mariusz; Zieliński, Krzysztof; Ingram, David (eds.). Transformation of Healthcare with Information Technologies. Studies in Health Technology and Informatics. Vol. 105. IOS Press. pp. 61–9. PMID 15718595.
- Tolksdorf, Robert; Bontas, Elena Paslaru (2004). "Organizing Knowledge in a Semantic Web for Pathology". Object-Oriented and Internet-Based Technologies. Lecture Notes in Computer Science. Vol. 3263. pp. 115–56. S2CID 18006838.
- Potts, Steven J. (2009). "Digital pathology in drug discovery and development: Multisite integration". Drug Discovery Today. 14 (19–20): 935–41. PMID 19596461.
- Potts, Steven J.; Young, G. David; Voelker, Frank A. (2010). "The role and impact of quantitative discovery pathology". Drug Discovery Today. 15 (21–22): 943–50. PMID 20946967.
- Zwonitzer, R; Kalinski, T; Hofmann, H; Roessner, A; Bernarding, J (2007). "Digital pathology: DICOM-conform draft, testbed, and first results". Computer Methods and Programs in Biomedicine. 87 (3): 181–8. PMID 17618703.

![Ki67 stain calculation by QuPath in a pure seminoma, which gives a measure of the proliferation rate of the tumor. The colors represent the intensity of expression: blue-no expression, yellow-low, orange-moderate, and red-high expression.[14]](http://upload.wikimedia.org/wikipedia/commons/thumb/e/eb/Ki67_calculation_by_QuPath_in_a_pure_seminoma.png/295px-Ki67_calculation_by_QuPath_in_a_pure_seminoma.png)

![Breast cancer prediction by AI.[15]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4f/Histopathology_of_tumor_identification_by_AI.png/278px-Histopathology_of_tumor_identification_by_AI.png)