History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.
A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure
Ancient world
The modern concept of molecules can be traced back towards pre-scientific and Greek philosophers such as
Circa 450 BC Empedocles imagined fundamental elements (fire (![]() ), earth (
), earth (![]() ), air (
), air (![]() ), and water (
), and water (![]() )) and "forces" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties.[1]
)) and "forces" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties.[1]
In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass.[2] A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies.
The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe.
Greek atomism
The earliest views on the shapes and connectivity of atoms was that proposed by Leucippus, Democritus, and Epicurus who reasoned that the solidness of the material corresponded to the shape of the atoms involved. Thus, iron atoms are solid and strong with hooks that lock them into a solid; water atoms are smooth and slippery; salt atoms, because of their taste, are sharp and pointed; and air atoms are light and whirling, pervading all other materials.[3]
It was Democritus that was the main proponent of this view. Using analogies based on the experiences of the senses, he gave a picture or an image of an atom in which atoms were distinguished from each other by their shape, their size, and the arrangement of their parts. Moreover, connections were explained by material links in which single atoms were supplied with attachments: some with hooks and eyes others with balls and sockets (see diagram).[4]
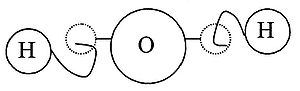
17th century
With the rise of scholasticism and the decline of the Roman Empire, the atomic theory was abandoned for many ages in favor of the various four element theories and later alchemical theories. The 17th century, however, saw a resurgence in the atomic theory primarily through the works of Gassendi, and Newton.
Among other scientists of that time Gassendi deeply studied ancient history, wrote major works about Epicurus natural philosophy and was a persuasive propagandist of it. He reasoned that to account for the size and shape of atoms moving in a void could account for the properties of matter. Heat was due to small, round atoms; cold, to pyramidal atoms with sharp points, which accounted for the pricking sensation of severe cold; and solids were held together by interlacing hooks.[5]
Newton, though he acknowledged the various atom attachment theories in vogue at the time, i.e. "hooked atoms", "glued atoms" (bodies at rest), and the "stick together by conspiring motions" theory, rather believed, as famously stated in "Query 31" of his 1704 Opticks, that particles attract one another by some force, which "in immediate contact is extremely strong, at small distances performs the chemical operations, and reaches not far from particles with any sensible effect."[6]
In a more concrete manner, however, the concept of aggregates or units of bonded atoms, i.e. "molecules", traces its origins to Robert Boyle's 1661 hypothesis, in his famous treatise The Sceptical Chymist, that matter is composed of clusters of particles and that chemical change results from the rearrangement of the clusters. Boyle argued that matter's basic elements consisted of various sorts and sizes of particles, called "corpuscles", which were capable of arranging themselves into groups.
In 1680, using the
18th century

An early precursor to the idea of bonded "combinations of atoms", was the theory of "combination via
These were lists, prepared by collating observations on the actions of substances one upon another, showing the varying degrees of affinity exhibited by analogous bodies for different reagents. These tables retained their vogue for the rest of the century, until displaced by the profounder conceptions introduced by CL Berthollet.
In 1738, Swiss physicist and mathematician Daniel Bernoulli published Hydrodynamica, which laid the basis for the kinetic theory of gases. In this work, Bernoulli positioned the argument, still used to this day, that gases consist of great numbers of molecules moving in all directions, that their impact on a surface causes the gas pressure that we feel, and that what we experience as heat is simply the kinetic energy of their motion. The theory was not immediately accepted, in part because conservation of energy had not yet been established, and it was not obvious to physicists how the collisions between molecules could be perfectly elastic.
In 1789,

19th century

Similar to these views, in 1803
Amedeo Avogadro created the word "molecule".[8] His 1811 paper "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies", he essentially states, i.e. according to Partington's A Short History of Chemistry, that:[9]
The smallest particles of gases are not necessarily simple atoms, but are made up of a certain number of these atoms united by attraction to form a single molecule.
Note that this quote is not a literal translation. Avogadro uses the name "molecule" for both atoms and molecules. Specifically, he uses the name "elementary molecule" when referring to atoms and to complicate the matter also speaks of "compound molecules" and "composite molecules".
During his stay in Vercelli, Avogadro wrote a concise note (memoria) in which he declared the hypothesis of what we now call
Avogadro developed this hypothesis to reconcile
In 1826, building on the work of Avogadro, the French chemist Jean-Baptiste Dumas states:
Gases in similar circumstances are composed of molecules or atoms placed at the same distance, which is the same as saying that they contain the same number in the same volume.
In coordination with these concepts, in 1833 the French chemist Marc Antoine Auguste Gaudin presented a clear account of Avogadro's hypothesis,[10] regarding atomic weights, by making use of "volume diagrams", which clearly show both semi-correct molecular geometries, such as a linear water molecule, and correct molecular formulas, such as H2O:
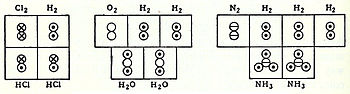
In two papers outlining his "theory of atomicity of the elements" (1857–58),
In 1856, Scottish chemist
In 1861, an unknown Vienna high-school teacher named
Loschmidt also suggested a possible formula for benzene, but left the issue open. The first proposal of the modern structure for benzene was due to Kekulé, in 1865. The cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale. Benzene presents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds:
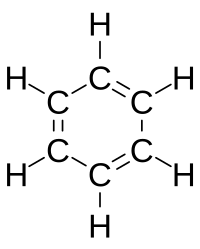
In 1865, German chemist
The basis of this model followed the earlier 1855 suggestion by his colleague
In 1864, Scottish organic chemist Alexander Crum Brown began to draw pictures of molecules, in which he enclosed the symbols for atoms in circles, and used broken lines to connect the atoms together in a way that satisfied each atom's valence.
The year 1873, by many accounts, was a seminal point in the history of the development of the concept of the "molecule". In this year, the renowned Scottish physicist James Clerk Maxwell published his famous thirteen page article 'Molecules' in the September issue of Nature.[15] In the opening section to this article, Maxwell clearly states:
An atom is a body which cannot be cut in two; a molecule is the smallest possible portion of a particular substance.
After speaking about the
In 1874,
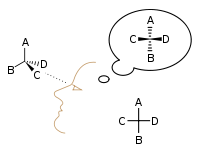
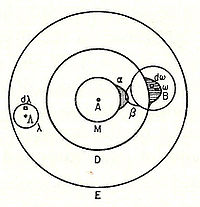
In 1898, Ludwig Boltzmann, in his Lectures on Gas Theory, used the theory of valence to explain the phenomenon of gas phase molecular dissociation, and in doing so drew one of the first rudimentary yet detailed atomic orbital overlap drawings. Noting first the known fact that molecular iodine vapor dissociates into atoms at higher temperatures, Boltzmann states that we must explain the existence of molecules composed of two atoms, the "double atom" as Boltzmann calls it, by an attractive force acting between the two atoms. Boltzmann states that this chemical attraction, owing to certain facts of chemical valence, must be associated with a relatively small region on the surface of the atom called the sensitive region.
Boltzmann states that this "sensitive region" will lie on the surface of the atom, or may partially lie inside the atom, and will firmly be connected to it. Specifically, he states "only when two atoms are situated so that their sensitive regions are in contact, or partly overlap, will there be a chemical attraction between them. We then say that they are chemically bound to each other." This picture is detailed below, showing the α-sensitive region of atom-A overlapping with the β-sensitive region of atom-B:[16]
20th century
In the early 20th century, the American chemist
Moreover, noting that a cube has eight corners Lewis envisioned an atom as having eight sides available for electrons, like the corner of a cube. Subsequently, in 1902 he devised a conception in which
In other words, electron-pair bonds are formed when two atoms share an edge, as in structure C below. This results in the sharing of two electrons. Similarly, charged ionic-bonds are formed by the transfer of an electron from one cube to another, without sharing an edge A. An intermediate state B where only one corner is shared was also postulated by Lewis.

Hence, double bonds are formed by sharing a face between two cubic atoms. This results in the sharing of four electrons.
In 1913, while working as the chair of the department of chemistry at the
On these views, in his famous 1916 article The Atom and the Molecule, Lewis introduced the "Lewis structure" to represent atoms and molecules, where dots represent
.
In Lewis' own words:
An electron may form a part of the shell of two different atoms and cannot be said to belong to either one exclusively.
Moreover, he proposed that an atom tended to form an ion by gaining or losing the number of electrons needed to complete a cube. Thus, Lewis structures show each atom in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another; occasionally, pairs of dots are used instead of lines. Excess electrons that form lone pairs are represented as pair of dots, and are placed next to the atoms on which they reside:
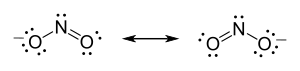
To summarize his views on his new bonding model, Lewis states:[19]
Two atoms may conform to the rule of eight, or the octet rule, not only by the transfer of electrons from one atom to another, but also by sharing one or more pairs of electrons...Two electrons thus coupled together, when lying between two atomic centers, and held jointly in the shells of the two atoms, I have considered to be the chemical bond. We thus have a concrete picture of that physical entity, that "hook and eye" which is part of the creed of the organic chemist.
The following year, in 1917, an unknown American undergraduate chemical engineer named
In 1927, the physicists Fritz London and Walter Heitler applied the new quantum mechanics to the deal with the saturable, nondynamic forces of attraction and repulsion, i.e., exchange forces, of the hydrogen molecule. Their valence bond treatment of this problem, in their joint paper,[20] was a landmark in that it brought chemistry under quantum mechanics. Their work was an influence on Pauling, who had just received his doctorate and visited Heitler and London in Zürich on a Guggenheim Fellowship.
Subsequently, in 1931, building on the work of Heitler and London and on theories found in Lewis' famous article, Pauling published his ground-breaking article "The Nature of the Chemical Bond"
Owing to these exceptional theories, Pauling won the 1954
In 1926, French physicist
In 1937, chemist
In 1951, physicist Erwin Wilhelm Müller invents the field ion microscope and is the first to see atoms, e.g. bonded atomic arrangements at the tip of a metal point.[23]
In 1968-1970 Leroy Cooper, PhD of the University of California at Davis completed his thesis which showed what molecules looked like. He used x-ray deflection off crystals and a complex computer program written by Bill Pentz of the UC Davis Computer Center. This program took the mapped deflections and used them to calculate the basic shapes of crystal molecules. His work showed that actual molecular shapes in quartz crystals and other tested crystals looked similar to the long envisioned merged various sized soap bubbles theorized, except instead of being merged spheres of different sizes, actual shapes were rigid mergers of more tear dropped shapes that stayed fixed in orientation. This work verified for the first time that crystal molecules are actually linked or stacked merged tear drop constructions. [citation needed]
In 1999, researchers from the
In 2009, researchers from
See also
- History of chemistry
- History of quantum mechanics
- History of thermodynamics
- History of molecular biology
- Kinetic theory of gases
- Atomic theory
References
- ISBN 978-1-4165-5477-6.
- ISBN 978-1-4165-5477-6.
- ISBN 1-86094-250-4.)
{{cite book}}: CS1 maint: multiple names: authors list (link - ISBN 0-300-00034-0. ASIN B0007EK5MM.
- ISBN 0-486-61053-5.)
{{cite book}}: CS1 maint: multiple names: authors list (link - ISBN 0-19-515040-6.)
{{cite book}}: CS1 maint: multiple names: authors list (link - ^ Lemery, Nicolas. (1680). An Appendix to a Course of Chymistry. London, pgs 14-15.
- ^ Ley, Willy (June 1966). "The Re-Designed Solar System". For Your Information. Galaxy Science Fiction. pp. 94–106.
- ^ Avogadro, Amedeo (1811). "Masses of the Elementary Molecules of Bodies". Journal de Physique. 73: 58–76.
- S2CID 143759556.
- ^ "Chemical Bonding Concept/Skills Development". intro.chem.okstate.edu. Retrieved 2023-01-01.
- ISBN 9780941901123.
- ^ Bader, A. & Parker, L. (2001). "Joseph Loschmidt", Physics Today, Mar.
- ^ Ollis, W. D. (1972). "Models and molecules". Proceedings of the Royal Institution of Great Britain. 45: 1–31.
- ^ Maxwell, James Clerk, "Molecules Archived 2007-02-09 at the Wayback Machine". Nature, September, 1873.
- ISBN 0-486-68455-5.
- ISBN 0-7382-0594-X.
- ^ Parson, A.L. (1915). "A Magneton Theory of the Structure of the Atom". Smithsonian Publication 2371, Washington.
- ^ "Valence and The Structure of Atoms and Molecules", G. N. Lewis, American Chemical Society Monograph Series, page 79 and 81.
- S2CID 119739102.
- .
- ^ Perrin, Jean, B. (1926). Discontinuous Structure of Matter, Nobel Lecture, December 11.
- ^ Mitch Jacoby, "Atomic Imaging Turns 50", Chemical & engineering News, 83:48, pp. 13–16, 28 November 2005
- S2CID 4424892.
- S2CID 5416065.
- ^ Single molecule's stunning image.
Further reading
- Partington, J.R. (1989). A Short History of Chemistry. Dover Publications, Inc. ISBN 0-486-65977-1.
- Atkins, Peter (2003). Atkins' Molecules, 2nd Ed. Cambridge University Press. ISBN 0-521-53536-0.
- Sargent, Ted (2006). The Dance of Molecules - How Nanotechnology is Changing our Lives. Thunder's Mouth Press. ISBN 1-56025-809-8.
- Scerri, Eric R. (2007). The Periodic Table, Its Story and Its Significance. Oxford University Press. ISBN 978-0-19-530573-9.
External links
- Geometric Structures of Molecules - Middlebury College
- Atoms and Molecules - McMaster University
- 3D Molecule Viewer - The Wileys Family
- Molecule of the Month - School of Chemistry, University of Bristol
- [1] - Eric Scerri's history & philosophy of chemistry website
Types
- Antibody Molecule - The National Health Museum
- 15 Types of Molecules - IUPAC Definitions
Definitions
- Molecule Definition - Frostburg State University (Department of Chemistry)
- Definition of Molecule - IUPAC
Articles
- Molecules Used to Make Nano-sized Containers - TRN Newswire
- Molecular Computer Processors - HP Labs
