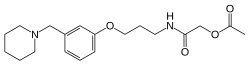
Roxatidine acetate
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Protein binding | 5–7% |
| Metabolism | Hepatic deacetylation Minor involvement of CYP2D6 and CYP2A6 |
| Elimination half-life | 5–7 hours |
| Excretion | Renal |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Roxatidine acetate is a specific and competitive
Pharmacodynamic studies showed that 150 mg of roxatidine acetate were optimal in suppressing gastric acid secretion, and that a single bedtime dose of 150 mg was more effective than a dose of 75 mg twice daily in terms of inhibiting nocturnal acid secretion.[1]
It was patented in 1979 and approved for medical use in 1986.[3] It is available in countries including China, Japan, Korea, Germany, Italy, the Netherlands, Greece and South Africa.[2]
References
- ^ S2CID 46973503.
- ^ a b BioSpectrum Bureau 1 November 2012 Sinhuan's generic heart drug gets production approval
- ISBN 9783527607495.
