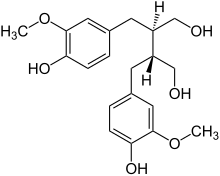
Secoisolariciresinol
| |
| Names | |
|---|---|
| IUPAC name
(8R,8′R)-3,3′-Dimethoxylignane-4,4′,9,9′-tetrol
| |
| Systematic IUPAC name
(2R,3R)-2,3-Bis[(4-hydroxy-3-methoxyphenyl)methyl]butane-1,4-diol | |
| Other names
(−)-Secoisolariciresinol
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.045.076 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H26O6 | |
| Molar mass | 362.422 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Secoisolariciresinol is an organic compound. It is classified as a lignan, i.e., a type of phenylpropanoid. It is present in some cereals, such as rye, and together with matairesinol has attracted much attention for its beneficial nutritional effects.[1]
Occurrence
The water extract of
Biomedical aspects
In the intestine the gut microflora can form secoisolariciresinol from the secoisolariciresinol diglucoside and it can then be further transformed into the enterolignan enterodiol. Epidemiological studies showed associations between secoisolariciresinol intake and decreased risk of cardiovascular disease are promising, but they are yet not well established, perhaps due to low lignan intakes in habitual Western diets. At the higher doses used in intervention studies, associations were more evident.[5][6]
