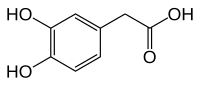
3,4-Dihydroxyphenylacetic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3,4-Dihydroxyphenyl)acetic acid | |
| Other names
2-(3,4-Dihydroxyphenyl)acetic acid
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
ECHA InfoCard
|
100.002.750 |
| KEGG | |
| MeSH | 3,4-Dihydroxyphenylacetic+Acid |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.148 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3,4-Dihydroxyphenylacetic acid (DOPAC) is a
catechol-O-methyl transferase (COMT), albeit in reverse order: MAO catalyzes dopamine to DOPAC, and COMT catalyzes DOPAC to HVA; whereas COMT catalyzes dopamine to 3-MT and MAO catalyzes 3-MT to HVA. The third metabolic end-product of dopamine is norepinephrine
(noradrenaline).
DOPAC can be oxidized by
levodopa treatment of Parkinson's disease. A MAO-B inhibitor such as selegiline or rasagiline can prevent this from happening.[citation needed
]

It can also be found in the bark of Eucalyptus globulus.[1]
This product has been synthesized (52% yield) from
References
- PMID 21761864.
- .
- ISBN 9781119991397.
