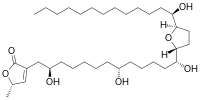
Annonacin
| |
| Names | |
|---|---|
| Preferred IUPAC name
(5S)-5-Methyl-3-[(2R,8R,13R)-2,8,13-trihydroxy-13-{(2R,5R)-5-[(1R)-1-hydroxytridecyl]oxolan-2-yl}tridecyl]furan-2(5H)-one | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C35H64O7 | |
| Molar mass | 596.890 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Annonacin is a chemical compound with toxic effects, especially in the nervous system, found in some fruits such as the paw paw, custard apples, soursop, and others from the family Annonaceae. It is a member of the class of compounds known as acetogenins. Annonacin-containing fruit products are regularly consumed throughout the West Indies for their traditional medicine uses.
Traditional medicine

Historically, plants and fruits of Annonaceae (particularly
It was discovered in Guadeloupe that atypical Parkinsonism was predominant in elderly males, who regularly consume annonacin-containing herbal teas.[3] Of 87 people with Parkinsonism transferred to one clinic between 1996 and 1998, 25% had Parkinson's, while 36% had progressive supranuclear palsy and 39% had atypical Parkinsonism.[3]
Neurotoxicity
Annonacin is a disabling and potentially lethal
The
Annonacin has been linked to the abnormally high incidence of progressive supranuclear palsy as well as atypical Parkinsonism in the Caribbean island of Guadeloupe where consumption of fruits, such as the soursop (Annona muricata), is common.[3] An average-sized soursop fruit contains 15 mg of annonacin, while a can of commercial nectar contains 36 mg and a cup of infusion, 140 μg.[7] Studies in rodents indicate that consumption of annonacin (3.8 and 7.6 mg per kg per day for 28 days) caused brain lesions consistent with Parkinson's disease.[8][9] An adult who consumes a fruit or can of nectar daily over the course of a year is estimated to ingest the same amount of annonacin that induced brain lesions in the rodents receiving purified annonacin intravenously.[7]
