C2-Symmetric ligands
In
Examples
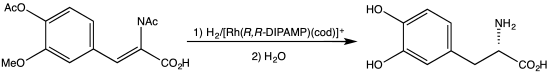
An early C2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry.[2] This ligand was used in the industrial production of L-DOPA.
Some classes of C2-symmetric ligands are called privileged ligands, which are ligands that are broadly applicable to multiple catalytic processes, not only a single reaction type.[3][4]
- Ligands and Complexes
-
DuPhos ligands are a class of C2-symmetric ligands for asymmetric hydrogenation.[6]
-
anticancer drug.
-
Jacobsen's epoxidation catalyst is a complex of a C2-symmetric salen-type ligand.
-
C2-symmetric diene ligand.[7]
-
Both bi- and tridentate bis(oxazoline) ligands are used in organic synthesis
-
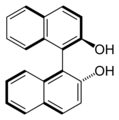
Both enantiomers of BINAP
-
BINOL, another binaphthalene-based ligand
-
DIPAMP, a diphosphine of historic significance
Mechanistic concepts
While the presence of any symmetry element within a ligand intended for asymmetric induction might appear counterintuitive, asymmetric induction only requires that the ligand be chiral (i.e. have no improper rotation axis). Asymmetry (i.e. absence of any symmetry elements) is not required. C2 symmetry improves the enantioselectivity of the complex by reducing the number of unique geometries in the transition states. Steric and kinetic factors then usually favor the formation of a single product.[1][8]
Chiral fence
Chiral ligands work by
Other C2-symmetric complexes
Many C2-symmetric complexes are known. Some arise not from C2-symmetric ligands, but from the orientation or disposition of high symmetry ligands within the coordination sphere of the metal. Notably,
Asymmetric ligands
Ligands containing atomic chirality centers such
See also
- Chiral anion catalysis
Further reading
- Desimoni, G.; Faita, G.; Jorgensen, K. A. (2006). "C2-Symmetric Chiral Bis(Oxazoline) Ligands in Asymmetric Catalysis". Chem. Rev. 106 (9): 3561–3651. PMID 16967916.
- Liu, X.; Lin, L.; Feng, X. (2011). "Chiral N,N'-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions". Acc. Chem. Res. 44 (8): 574–587. PMID 21702458.
- Evans, D. A.; Kozlowski, M. C.; Murry, J. A.; Burgey, C. S.; Campos, K. R.; Connell, B. T.; Staples, R. J. (1999). "C2-Symmetric Copper(II) Complexes as Chiral Lewis Acids. Scope and Mechanism of Catalytic Enantioselective Aldol Additions of Enolsilanes to (Benzyloxy)Acetaldehyde". J. Am. Chem. Soc. 121 (4): 669–685. .
- Gao, J.-X.; Ikariya, T.; Noyori, R. (1996). "A Ruthenium(II) Complex with a C2-Symmetric Diphosphine/Diamine Tetradentate Ligand for Asymmetric Transfer Hydrogenation of Aromatic Ketones". Organometallics. 15 (4): 1087–1089. .
- Pye, P. J.; Rossen, K.; Reamer, R. A.; Tsou, N. N.; Volante, R. P.; Reider, P. J. (1997). "New Planar Chiral Bisphosphine Ligand for Asymmetric Catalysis: Highly Enantioselective Hydrogenations under Mild Conditions". J. Am. Chem. Soc. 119 (26): 6207–6208. .

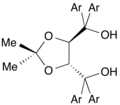
![The C2-symmetric diphosphine DIOP is historically significant.[5]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/95/%28S%2CS%29-DIOP.svg/120px-%28S%2CS%29-DIOP.svg.png)
![DuPhos ligands are a class of C2-symmetric ligands for asymmetric hydrogenation.[6]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/2c/DuPhos_ligands.svg/120px-DuPhos_ligands.svg.png)


![C2-symmetric diene ligand.[7]](http://upload.wikimedia.org/wikipedia/commons/thumb/0/0d/HayashiChiralNBD.svg/120px-HayashiChiralNBD.svg.png)