Ligandrol
 | |
| Clinical data | |
|---|---|
| Other names | LGD4033; VK5211; VK-5211; Ligandrol; Anabolicum |
| Routes of administration | By mouth[1][2] |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 24–36 hours[3][2][4] |
| Identifiers | |
| |
JSmol) | |
| |
| |
LGD-4033, also known by the developmental code name VK5211 and by the black-market name Ligandrol, is a
Known possible
LGD-4033 was first described in 2010.[12][4] It is less clinically studied than other SARMs like enobosarm, with only a few small clinical trials having been conducted and reported.[14][11][9][2][8] LGD-4033 has not yet completed clinical development or been approved for any use.[5][10][3] As of 2023, it is in phase 2 clinical trials for the treatment of hip fracture and muscle atrophy.[5] LGD-4033 was developed by Ligand Pharmaceuticals, and is now being developed by Viking Therapeutics.[5]
Aside from its development as a potential
Medical uses
LGD-4033 is not approved for any medical use and is not available as a licensed
Side effects
LGD-4033 and other SARMs are largely uncharacterized in terms of their potential for
The United States Food and Drug Administration (FDA) claims that "liver toxicity, adverse effects on blood lipid levels, and a potential to increase the risk of heart attack and stroke" are among the potential adverse health effects of SARMs including LGD-4033.[20]
Overdose
LGD-4033 has been assessed in clinical trials at single doses ranging from 0.1 to 22 mg and at repeated doses ranging from 0.1 to 2 mg/day for 3 to 12 weeks.[11] The drug sold via black-market Internet suppliers and used non-medically is often taken at much higher doses than those used in repeated-dose clinical trials (e.g., 5–10 mg/day), with unknown adverse effects and risks.[3][9][11]
Pharmacology
Pharmacodynamics
LGD-4033 is a
LGD-4033 has shown robust selectivity for stimulation of the levator ani muscle relative to stimulation of the prostate in rats.[12] At the highest assessed dose in castrated male rats, levator ani weight was increased to around 140% of that of gonadally intact controls, whereas prostate weight was only increased to around 45% of that of intact controls.[13] The tissue selectivity of LGD-4033 was independent of local tissue drug concentration, suggesting that its selectivity was intrinsic.[12][13] The muscle-stimulating effects of LGD-4033 have also been confirmed in humans in preliminary clinical trials.[10][26] The data also allow comparison between different SARMs and other AR agonists.[10][26] In a phase 1 clinical trial in 76 healthy young men, 1 mg/day LGD-4033 increased lean body mass by 1.2 kg after 3 weeks of treatment.[10][26][2] For comparison, enobosarm, another SARM, increased lean body mass by 1.3 kg at a dose of 3 mg/day after 12 weeks in healthy elderly men and postmenopausal women.[2][26][27] It was concluded that the employed dose of LGD-4033 produced similar increases in lean body mass compared to enobosarm despite a substantially shorter treatment period.[2] In a phase 2 clinical trial in 108 women and men with hip fracture, LGD-4033 increased lean body mass by 4.8% at 0.5 mg/day, 7.2% at 1 mg/day, and 9.1% at 2 mg/day after 12 weeks of treatment.[8] For comparison, lean body mass with enobosarm 3 mg/day after the same time period of 12 weeks increased by about 0.30% at 0.1 mg/day, 0.40% at 0.3 mg/day, 1.2% at 1 mg/day, and 3.1% at 3 mg/day, with only the latter change achieving statistical significance.[27] Relative to SARMs, supraphysiological doses of testosterone (300–600 mg/week intramuscular testosterone enanthate) over similar timeframes, like 20 weeks, have been found to result in lean body mass gains of 5 to 8 kg in healthy young men.[28][3][29]
In addition to selectivity for muscle and bone over the prostate gland, LGD-4033 has also been stated by Ligand Pharmaceuticals researchers to have reduced strength in the sebaceous glands.[12][4] Reduced activity in stimulating sebaceous gland formation, to about 30 to 50% of that produced by DHT at doses with similar anabolic potency in rats, has also been reported for certain other SARMs, like the steroidal agents TFM-4AS-1 and MK-0773.[12] In addition, enobosarm and MK-0773 have been reported to limitedly stimulate the sebaceous glands in small short-term clinical studies in women.[30][27][31]
Pharmacokinetics
LGD-4033 showed
Chemistry
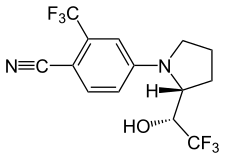
LGD-4033 is a
LGD-4033 is a
History
The predecessor of LGD-4033,
LGD-4033 was developed by Ligand Pharmaceuticals and was first described in the literature in 2010.
Society and culture
Regulatory information
In the United States, LGD-4033 is an Investigational New Drug and is not approved for any medical use.[5]
Non-medical use
Though not an approved drug, LGD-4033 (Ligandrol) has been sold on the black market in countries where it is classified as an illegal substance.[42][43] Along with enobosarm (ostarine; GTx-024, S-22), andarine (GTx-007; S-4), and vosilasarm (RAD140; "testolone"), LGD-4033 is one of the most popular and common non-medically-used SARMs.[9][44] Many products sold online that are purported to be LGD-4033 either contain none or contain other unrelated substances, and doses are also frequently not as labeled.[3][16] Social media has played an important role in facilitating the widespread non-medical use of SARMs.[17]
On 23 October 2017, a nutritional supplement company in Missouri called Infantry Labs was warned by the FDA that the distribution of two of its products violated the
Also on 23 October 2017, the FDA sent a warning letter to a New Jersey company called Panther Sports Nutrition. The company's marketing approach for the product was similar to that of the Infantry Labs case, and the product was advertised as a "mass builder" and "physique enhancing agent".[45]
Doping in sport
LGD-4033 is on the
List of doping cases
In 2015, the quarterback of the Florida Gators, Will Grier, was suspended for testing positive for LGD-4033, a claim that the University of Florida denies.[49]
In 2017,
In 2019, Australian swimmer Shayna Jack tested positive for LGD-4033. She denies knowingly taking the substance.[51]
In August 2019, it came to light that Canadian sprint canoeist
In January 2020, Chilean ATP tennis singles competitor Nicolás Jarry tested positive for both LGD-4033 and stanozolol. He protested at the time that the multi-vitamins from Brazil that he took on the advice of an unnamed doctor were contaminated.[54]
On 3 September 2022, sprinter Nzubechi Grace Nwokocha was provisionally suspended for the use of banned substances enobosarm and LGD-4033[55] by the Athletics Integrity Unit (AIU).
On 23 January 2024, Tristan Thompson was suspended for 25 games by the NBA for testing positive for ibutamoren and LGD-4033.[56]
On 12 March 2024, curler Briane Harris was provisionally suspended for up to four years after testing positive for LGD-4033. She denies this after being tested by doping control officers on Jan. 24 and notified of her positive test on Feb. 15. A second sample, called the B sample, also confirmed the positive test. She plans to appeal the ban to the Court of Arbitration for Sport, arguing she was unknowingly exposed to it through bodily contact. [57]
On 15 March 2014 cyclist Christos Volikakis was informed of an Adverse Analytical Finding on a re-analysis of a sample from the 2016 Rio Olympics. The athlete has since requested an analysis of the B sample.[58]
Research
Oral administration of LGD-4033 to cynomolgus monkeys at daily doses varying from 0 to 75 mg/kg over 13 weeks demonstrated significant body weight gain in both males and females. After 48 days, the 75 mg/kg dose testing was halted due to toxicity concerns, but this did not negatively impact development of the drug as this dose is significantly higher than the doses being utilized in a phase 2 clinical trial.[59]
Two
A
As of 2023, LGD-4033 has been less studied than other SARMs like enobosarm, with only three small phase 1 clinical trials and one phase 2 trial, or a total of four clinical studies, having been conducted and reported.[14][11][9][2][8]
References
- ^ S2CID 8104778.
- ^ PMID 22459616.
- ^ S2CID 225049089.
- ^ ISSN 0918-8959.
LGD-4033 is a potent SARM that binds the human androgen receptor with Kd =0.9 nM. In animal models, it has anabolic effects on skeletal muscle and bone, but spares prostate, sebaceous glands, and female genitalia. In a double-blind, placebo-controlled, first-in-human Phase I trial, ascending single oral doses of LGD-4033 ranging from 0.1 mg to 22 mg were administered to healthy males. LGD4033 was safe and well tolerated up to the highest tested dose with no serious adverse events reported. LGD-4033 exhibited dose-proportional, sustained systemic exposure (AUC0-48hr: 24 to 7000 ng. hr/ mL for 0.1 and 22 mg doses, respectively). The elimination half-life (t1/2) was 31 hrs, indicating LGD-4033 is amenable for once daily dosing. PK-PD studies were conducted in orchiectomized (ORDX) rats, a model of androgen action, to determine the LGD-4033 efficacious exposure level. Subcutaneous minipumps were used to mimic the 10-fold longer t1/2 in humans vs. rats. A dose that produced an AUC of 80 ng. hr/mL restored the atrophied muscle mass of ORDX rats to the eugonadal level (270% increase in levator ani muscle weight with LGD-4033 vs. vehicle) and reduced the elevated luteinizing hormone level of ORDX rats by 98%. The efficacious range predicted by the preclinical model will be achieved by repeated daily doses ca. 0.25 mg in humans. Conclusion: LGD-4033 is a well-tolerated and highly tissue-specific, potential new treatment for sarcopenia (e.g., cancer cachexia or the frail elderly) and osteoporosis that is predicted to be effective using low, daily oral doses. A Phase I multi-dose study is in progress.
- ^ a b c d e f g h i j k "VK 5211 - AdisInsight".
- ^ PMID 36479151.
- ^ S2CID 209246318.
Other molecules have been developed including LGD-4033 which increased muscle mass and strength in healthy males after 3 weeks (Basaria et al. 2013) [...] Recently, a phase 2 trial on the agent VK211 demonstrated dose-dependent increases in lean body mass, and improvements in physical performance in patients who had sustained hip fracture (Ristic et al. 2018). Whilst SARMs hold great promise as anabolic agents that may offer an effective therapy for osteosarcopenia, long-term side effects of these agents are unknown, studies are generally small and of short duration. Regulation of these products poses immense challenges with their high uptake on the black market and via the internet as performance-enhancing, body-building agents, which may overshadow their potential mainstream application in disorders of aging.
- ^ PMID 30444937.
Introduction Hip fractures are a leading cause of disability and morbidity in older people. Post-facture, an increased catabolic state often leads to loss of muscle, which can impair balance and endurance, potentially increasing the risk of further injury. Anabolic steroids have been shown to improve muscle mass in certain settings. Selective androgen receptor modulators (SARMs) could be similarly effective in older patients who have suffered muscle loss following hip fracture, while potentially avoiding undesired side effects associated with broad-acting anabolic agents. VK5211 is a novel, non-steroidal, orally available SARM that has been shown to improve muscle mass and bone mineral density in animal models. In humans, a prior Phase 1 study demonstrated increases in lean body mass after 21 days of dosing. Purpose A 12 week study was conducted to assess the safety and efficacy of VK5211 in patients who had suffered a hip fracture. Methods A randomized, double-blind, placebo-controlled, multicenter, international Phase 2 trial was conducted to evaluate VK5211 in patients recovering from hip fracture. Patients were randomized to receive daily oral VK5211 doses of 0.5 mg, 1.0 mg, 2.0 mg, or placebo, for 12 weeks. The primary endpoint evaluated change from baseline in lean body mass, less head, in patients receiving VK5211 compared with placebo. Secondary and exploratory endpoints included changes in appendicular lean mass, bone density, and functional performance. Results A total of 108 patients were randomized (83 F, 25 M; mean age 77). Patients receiving VK5211 demonstrated significant increases in lean body mass, less head, after 12 weeks. Placebo-adjusted increases were 4.8% at 0.5 mg, 7.2% at 1.0 mg, and 9.1% at 2.0 mg (p < 0.005 for each). The proportions of patients experiencing at least a 2.0 kg increase were 14% with placebo, 57% at 0.5 mg, 65% at 1.0 mg, and 81% at 2.0 mg (p < 0.01 for each). Patients receiving VK5211 demonstrated improvement in certain measures of functional performance, including the 6-minute walk test and short physical performance battery (these endpoints were not powered for significance). The rates of adverse events were similar in cohorts receiving VK5211 as compared with placebo, and no drug-related SAEs were observed in VK5211-treated patients. Conclusion VK5211 was well-tolerated and produced improvements in lean body mass in hip fracture patients following 12 weeks of dosing. Further evaluation in this setting is warranted.
- ^ PMID 37571268.
- ^ S2CID 219174372.
- ^ PMID 37218811.
- ^ S2CID 2584722.
- ^ a b c d e f g Vajda EG, Marschke K, van Oeveren A, Zhi L, Chang WY, López FJ, et al. (2009), "LGD-4033 builds muscle and bone with reduced prostate activity and may be beneficial in age-related frailty" (PDF), Proceedings of the 62nd Annual Meeting of the Gerontology Society of America, November 11-21, 2009, Atlanta, Georgia
- ^ a b c d e "VK5211". Viking Therapeutics.
- ^ a b "Prohibited List". World Anti Doping Agency. 2014-07-22.
- ^ PMID 29183075.
- ^ PMID 35574698.
- PMID 33672087.
- PMID 38059982.
- ^ a b "WARNING LETTER Infantry Labs LLC MARCS-CMS 535333 — OCT 23, 2017". FDA. 23 October 2017.
- S2CID 43199715.
- ^ S2CID 96279138.
- ^ PMID 18500378.
- PMID 33042018.
- S2CID 259352099.
- ^ S2CID 35120033.
- ^ PMID 22031847.
- PMID 19357508.
At the doses that have been tested, the first generation SARMs induce modest gains in lean body mass in healthy volunteers, which are nowhere near the much greater gains in skeletal muscle mass reported with supraphysiological doses of testosterone. The modest gains of 1.0 to 1.5 kg in fat-free mass with first generation SARMs over 4–6 weeks should be contrasted with the 5–7 kg gains in fat-free mass with 300 and 600 mg doses of testosterone enanthate. However, it is possible that next generation of SARM molecules will have greater potency and selectivity than the first generation SARMs.
- S2CID 2344757.
The administration of the GnRH agonist plus graded doses of testosterone resulted in mean nadir testosterone concentrations of 253, 306, 542, 1,345, and 2,370 ng/dl at the 25-, 50-, 125-, 300-, and 600-mg doses, respectively. Fat-free mass increased dose dependently in men receiving 125, 300, or 600 mg of testosterone weekly (change +3.4, 5.2, and 7.9 kg, respectively). The changes in fat-free mass were highly dependent on testosterone dose (P = 0.0001) and correlated with log testosterone concentrations (r = 0.73, P = 0.0001).
- S2CID 23450056.
- PMID 20356837.
- PMID 34734312.
- S2CID 263465407.
- PMID 26767942.
- PMID 27168428.
- ^ "Ligandrol". PubChem. U.S. National Library of Medicine.
- ^ "Ligandrol". DrugBank.
- ^ PMID 9925725.
- ^ PMID 16841196.
- ISBN 978-1-139-00335-3.
- ^ a b "Viking Therapeutics Initiates Phase 2 Trial of VK5211 in Patients Recovering From Hip Fracture". FierceBiotech. Questex LLC. 21 November 2016.
- PMID 26767942.
- S2CID 111542.
- PMC 10392554.
- ^ "WARNING LETTER Panther Sports Nutrition MARCS-CMS 535341 — OCT 23, 2017". FDA. 23 October 2017.
- PMID 27168428.
- PMID 26044265.
- PMID 29334634.
- ^ Trahan K (12 October 2015). "Florida starting QB Will Grier suspended for at least 2015 after taking banned substance". SB Nation. Retrieved 20 October 2015.
- ^ "NBA bans Joakim Noah 20 games for drug violation". Fox Sports. March 25, 2017. Archived from the original on 26 October 2019.
- ^ Maasdorp J (July 28, 2019). "Shayna Jack reveals banned substance LGD-4033 was behind her doping suspension from swimming". Australian Broadcasting Corporation. Retrieved July 28, 2019.
- ^ "Canada's Vincent Lapointe reveals she tested positive for muscle-building substance". CBC. 20 August 2019.
- ^ "Dopage : Laurence Vincent Lapointe blanchie et soulagée" [Doping: Laurence Vincent Lapointe cleared and relieved]. Radio Canada (in French). 27 January 2020.
- ^ Briggs S (15 January 2020). "Wimbledon doubles champion Robert Farah fails drugs test". Telegraph Media Group Limited.
- ^ "Commonwealth gold medallist Nwokocha provisionally suspended for doping". Reuters. 2022-09-03. Retrieved 2023-05-09.
- ^ "Cavs' Tristan Thompson suspended 25 games without pay by NBA". National Basketball Association. January 23, 2024. Retrieved January 23, 2024.
- ^ Heroux D. "Curler Briane Harris faces 4-year suspension after testing positive for banned substance, plans to appeal". CBC Sports. Retrieved 12 March 2024.
- ^ Weislo L (15 March 2024). "Greek track sprinter positive in re-analysis of 2016 Olympics samples". Cyclingnews. Retrieved 18 March 2024.
- ^ Bautz D (21 November 2016). "VKTX: Additional Preclinical Data Shows Robust and Durable Weight Gain for VK5211-Treated Primates". Yahoo. Yahoo, Inc.
