Magnus's green salt
| |
| |
| Names | |
|---|---|
| IUPAC name
Tetraammineplatinum(II) tetrachloroplatinate(II)
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.034.078 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
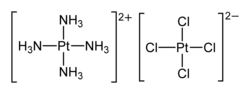
| [Pt(NH3)4][PtCl4] | |
| Molar mass | 600.09 g/mol |
| Appearance | green solid |
| Density | 3.7 g/cm3 |
| Melting point | 320 °C (608 °F; 593 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
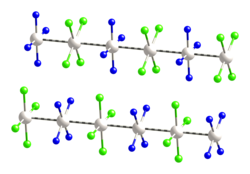
Magnus's green salt is the inorganic compound with the formula [Pt(NH3)4][PtCl4]. This salt is named after Heinrich Gustav Magnus, who, in the early 1830s, first reported the compound. The compound is a linear chain compound, consisting of a chain of platinum atoms. It is dark green, which is unusual for platinum compounds.
Structure
This species has attracted interest in materials chemistry and solid-state physics because of its one-dimensional structure. It contains a chain of alternating [PtCl4]2− anions and [Pt(NH3)4]2+ cations, in which the platinum atoms are separated by 3.25 Å.[1] It is a semiconductor.
Preparation
The compound may be prepared by combining aqueous solutions of [Pt(NH3)4]2+ and [PtCl4]2−, which gives a deep green solid precipitate.
Related compounds
Magnus's green salt has the same
Soluble analogues of Magnus's green salt can be prepared by replacing the ammonia with ethylhexylamine.[5][6]
The corresponding palladium compound ([Pd(NH3)4][PdCl4]) is known as "Vauquelin’s salt".
History
Magnus's green salt was one of the first examples of a metal ammine complex.
References
- .
- ISBN 978-0-470-13233-3.
- PMID 24437378.
- PMID 24283498.
- .
- .
