Oxaliplatin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eloxatin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607035 |
| License data |
|
Intravenous | |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Complete |
| Elimination half-life | ~10 – 25 minutes[4] |
| Excretion | Kidney |
| Identifiers | |
| |
JSmol) | |
| |
| | |
Oxaliplatin, sold under the brand name Eloxatin among others, is a
Common side effects include
Oxaliplatin was patented in 1976 in Japan and approved for medical use in 1996 in Europe.[8] It is on the 2023 World Health Organization's List of Essential Medicines.[9]
Medical uses
Oxaliplatin is used for treatment of
Advanced colorectal cancer
Oxaliplatin by itself has modest activity against advanced colorectal cancer.
Adverse effects
Side-effects of oxaliplatin treatment can potentially include:
- Neurotoxicity leading to chemotherapy-induced peripheral neuropathy, a progressive, enduring and often irreversible tingling numbness, intense pain and hypersensitivity to cold, beginning in the hands and feet and sometimes involving the arms and legs, often with deficits in proprioception.[16] This chronic neuropathy may also be preceded by a transient acute neuropathy occurring at the time of infusion and associated with excitation of voltage-gated Na+ channels.[17][18]
- Fatigue
- Nausea, vomiting, or diarrhea
- Neutropenia (low number of a type of white blood cells)
- Ototoxicity (hearing loss)
- Extravasation if oxaliplatin leaks from the infusion vein it may cause severe damage to the connective tissues.
- Hypokalemia (low blood potassium), which is more common in women than men[19]
- Persistent hiccups[20]
- Rhabdomyolysis[21]
In addition, some patients may experience an allergic reaction to platinum-containing drugs. This is more common in women.[19]
Oxaliplatin has less ototoxicity and nephrotoxicity than cisplatin and carboplatin.[16]
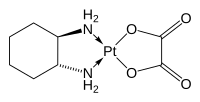
Structure and mechanism
The compound features a
According to
History
Oxaliplatin was first synthesized in 1978 at
Patent information
Eloxatin was covered by patent numbers 5338874 (expired 7 April 2013), 5420319 (expired 8 August 2016), 5716988 (expired 7 August 2015) and 5290961 (expired 12 January 2013) (see Electronic Orange Book patent info for Eloxatin).[30] Exclusivity code I-441, which expired on 4 November 2007, is for use combination with infusional 5-FU/LV for adjuvant treatment stage III colon cancer patients who have undergone complete resection primary tumor-based on improvement in disease free survival with no demonstrated benefit overall survival after 4 years. Exclusivity code NCE, New Chemical Entity, expired on 9 August 2007.[30]
References
- FDA. Retrieved 22 October 2023.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ "Eloxatin- oxaliplatin injection, solution, concentrate". DailyMed. 22 October 2019. Retrieved 26 May 2022.
- S2CID 1068099.
- ^ a b c d e f "Oxaliplatin". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ PMID 29632935.
- ^ PMID 26113607.
- ISBN 9783527607495. Archivedfrom the original on 20 December 2016.
- hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "FOLFOX". National Cancer Institute. 18 September 2009. Retrieved 26 May 2022.
- ^ "CAPOX". National Cancer Institute. 4 April 2012. Retrieved 26 May 2022.
- ^ "XELOX". National Cancer Institute. 6 January 2012. Retrieved 26 May 2022.
- ISBN 9780080552323.
- PMID 9704726.
- PMID 10944126.
- ^ PMID 16806962.
- PMID 16231011.
- PMID 29649985.
- ^ S2CID 33026126.
- ^ "Oxaliplatin Side Effects". Drugs.com. Archived from the original on 5 September 2014. Retrieved 5 September 2014.
- ^ "Eloxatin information". mein.sanofi.de (in German). Archived from the original on 27 August 2016. Retrieved 15 June 2016.
- PMID 24415827.
- PMID 14756144.
- ISBN 9780080965291.
- ISBN 978-0-323-04971-9.
- ^ "Eloxatin FDA Approval History". Drugs.com.
- ^ "Generic Eloxatin availability". Drugs.com. Archived from the original on 7 June 2013. Retrieved 19 April 2014.
- ^ "Hospira Announces U.S. Re-Launch Of Generic Oxaliplatin Injection" (Press release). Archived from the original on 24 September 2015. Retrieved 25 August 2015.
- ^ "Top 10 best-selling cancer drugs: Eloxatin–$1.2 billion". FiercePharma. 15 May 2012. Archived from the original on 21 April 2014. Retrieved 20 April 2014.
- ^ a b "Patent and Exclusivity Search Results from query on Appl No 021759 Product 001 in the OB_Rx list". Orange Book. U.S. Food and Drug Administrartion. Archived from the original on 26 September 2007.. Accessed on: 22 July 2007.
Further reading
- Graham J, Mushin M, Kirkpatrick P (January 2004). "Oxaliplatin" (PDF). Nature Reviews. Drug Discovery. 3 (1): 11–12. PMID 14756144. Archived from the original(PDF) on 8 November 2004. Retrieved 19 July 2005.
External links
- "Oxaliplatin". National Cancer Institute. 5 October 2006.
