Neural tissue engineering
This article needs to be updated. The reason given is: There have been advances made in several topics mentioned. (September 2019) |
Neural tissue engineering is a specific sub-field of
Introduction
There are two parts of the nervous system: the central nervous system (CNS) and the peripheral nervous system (PNS). General body functions are supervised by the central nervous system (CNS), which includes the brain and spinal cord. The PNS delivers motor signals to control body activities and receives sensory data from the CNS. The PNS It is made up of nerve fibers arranged into nerves. The PNS's autonomic nervous system (ANS), whose sympathetic and parasympathetic branches preserve homeostasis, regulates involuntary physiological functions.[1]
The "fight-or-flight" reaction is triggered by the
Neuroimmune Interactions The
Neuroimmune interplays have possible therapeutical approaches[9] Novel approaches focusing on neuroimmune interactions may alter the course of the disease or reduce symptoms. Targeting neuroimmune pathways is a holistic approach that seeks to affect both immune responses and brain functioning. The term "acupuncture" refers to the ancient Chinese medical technique of gently stimulating nociceptors and receptors with tiny needles inserted into certain body sites in order to treat various ailments, including pain and inflammation.[10] The
Tissue Engineering The need for neural
Recent research into creating miniature cortexes, known as
Another situation that calls for implanting of foreign tissue is use of recording
CNS
Causes of CNS injury
There are three main causes of CNS injury:
CNS treatments and research
Implantation of stem cells to the injury site
One method to treat CNS injury involves culturing stem cells
Alternatively, these stem cells can act as carriers for other therapies, though the positive effects of using stem cells as a delivery mechanism has not been confirmed. Direct stem cell delivery has an increased beneficial effect if they are directed to be neuronal cells in vitro. This way, the risks associated with undirected stem cells are decreased; additionally, injuries that do not have a specific boundary could be treated efficiently.[16]

Delivery of molecules to the injury site
Molecules that promote the regeneration of neural tissue, including
Implantation of neural tissue developed in vitro
A third method for treating CNS injuries is to artificially create tissue outside of the body to implant into the injury site. This method could treat injuries that consist of large cavities, where larger amounts of neural tissue needs to be replaced and regenerated. Neural tissue is grown in vitro with neural stem or
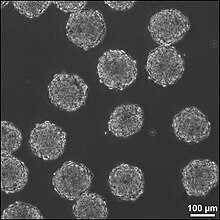
There are 6 different types of scaffolds that are being researched to use in this method for treating neural tissue injury:
- Liquid hydrogels are cross-linkedhydrophobic polymer chains, and the neural stem cells are either grown on the surface of the gel or integrated into the gel during cross-linking of the polymer chains. The major drawback of liquid hydrogels is there is limited protection of the cells that are transplanted.
- Supportive scaffolds are made from solid bead-shaped or microporous structures, and can act as carriers for the transplanted cells or for the growth factors that the stem cells secrete when they are differentiating. The cells adhere to the surface of the matrix in 2D layers. The supportive scaffolds are easily transplanted into the brain injury site because of the scaffold size. They provide a matrix promoting cell adhesion and aggregation, thus increasing increased healthy cell culture.
- Aligning scaffolds can be silk-based, polysaccharide-based, or based on other materials such as a collagen-rich hydrogel. These gels are now enhanced with micro-patterns on the surface for the promotion of neuronal outgrowths. These scaffolds are primarily used for regeneration that needs to occur in a specific orientation, such as in spinal cord injuries.
- Integrative scaffolds are mainly used to protect the transplanted cells from mechanical forces that they are exposed to in the process of implantation into the site of the injury. These scaffolds also decrease the likelihood of having the inflammatory cells located at the site of the injury migrate into the scaffold with the stem cells. Blood vessels have been observed to grow through the scaffold, thus the scaffold and cells are being integrated into the host tissue.
- A combination of engineered scaffolds presents an option for a 3D scaffold that can have both the necessary patterns for cell adhesion and the flexibility to adapt to the ever changing environment at the injury site. Decellularized ECM scaffolds is an option for scaffolds because they more closely mimc the native tissue, but these scaffolds can only currently be harvested from amputations and cadavers.[16]
These 3D scaffolds can be fabricated using particulate leaching, gas foaming, fiber bonding, solvent casting, or electrospinning techniques; each technique creates a scaffold with different properties than the other techniques.[22]
Incorporation success of 3D scaffolds into the CNS has been shown to depend on the stage at which the cells have differentiated. Later stages provide a more efficient implantation, while earlier staged cells need to be exposed to factors that coerce the cells to differentiate and thus respond appropriately to the signals the cells will receive at the CNS injury site.[23] Brain-derived neurotrophic factor is a potential co-factor to promote functional activation of ES cell-derived neurons into the CNS injury sites.[24]
PNS
Causes of PNS injury
Trauma to the PNS can cause damage as severe as a severance of the nerve, splitting the nerve into a
PNS treatments and research
Surgical reconnection
One method to treat PNS injury is surgical reconnection of the severed nerve by taking the two ends of the nerve and suturing them together. When suturing the nerves together, the fascicles of the nerve are each reconnected, bridging the nerve back together. Though this method works for severances that create a small gap between the
Tissue grafts
Tissue grafts utilize nerves or other materials to bridge the two ends of the severed nerve. There are three categories of tissue grafts: autologous tissue grafts, nonautologous tissue grafts, and acellular grafts.
Autologous tissue grafts transplant nerves from a different part of the body of the patient to fill the gap between either end of the injured nerve. These nerves are typically cutaneous nerves, but other nerves have been researched as well with encouraging results. These autologous nerve grafts are the current gold standard for PNS nerve grafting because of the highly biocompatible nature of the autologous nerve graft, but there are issues concerning harvesting the nerve from the patients themselves and being able to store a large amount of autologous grafts for future use.
Nonautologous and
Guidance
Natural-based materials are modified scaffolds stemming from
.Neuroimmune Enhancement Through EVs
Difficulty of research
Because there are so many factors that contribute to the success or failure of neural tissue engineering, there are many difficulties that arise in using neural tissue engineering to treat CNS and PNS injuries. First, the therapy needs to be delivered to the site of the injury. This means that the injury site needs to be accessed by surgery or drug delivery. Both of these methods have inherent risks and difficulties in themselves, compounding the problems associated with the treatments. A second concern is keeping the therapy at the site of the injury. Stem cells have a tendency to migrate out of the injury site to other sections of the brain, thus the therapy is not as effective as it could be as when the cells stay at the injury site. Additionally, the delivery of stem cells and other morphogens to the site of injury can cause more harm than good if they induce tumorigenesis, inflammation, or other unforeseen effects. Finally, the findings in laboratories may not translate to practical clinical treatments. Treatments are successful in a lab, or even an animal model of the injury, may not be effective in a human patient.[30]
Related research
Modeling brain tissue development in vitro
Two models for brain tissue development are
Targeted reinnervation
Targeted reinnervation is a method to reinnervate the neural connections in the CNS and PNS, specifically in paralyzed patients and amputees using prosthetic limbs. Currently, devices are being investigated that take in and record the electrical signals that are propagated through neurons in response to a person's intent to move. This research could shed light on how to reinnervate the neural connections between severed PNS nerves and the connections between the transplanted 3D scaffolds into the CNS.[21]
References
- ^ PMID 37767094.
- PMID 30263032.
- PMID 33028343.
- PMID 33527083.
- PMID 30294960.
- PMID 32144196.
- ^ S2CID 250361701– via PubMed.
- PMID 33677834.
- PMID 37153559.
- PMID 31680841.
- ^ PMID 29844694.
- PMID 35454883.
- PMID 35232750.
- PMID 35396067.
- S2CID 4302192.
- ^ S2CID 42424867.
- . Retrieved 2017-05-22 – via ResearchGate.
- ^ PMID 14527315.
- ^ PMID 19597331.
- ^ PMID 23995685.
- ^ a b c Tenore, Francesco; Vogelstein, Jacob (2011). "Revolutionizing Prothetics: Devices for Neural Integration". Johns Hopkins APL Technical Digest. 30 (3): 230–39.
- S2CID 205620361.
- S2CID 12291422.
- PMID 16293594.
- PMID 31311206.
- PMID 34204903.
- ^ PMID 35550636.
- S2CID 235076563.
- PMID 30866785.
- ^ LaPlaca, Michele (3 October 2013). "Personal Interview".
- S2CID 2611446.
